Pharmaceutical composition of freeze-dried powder injection containing caspofungin
A freeze-dried powder injection, caspofungin technology, applied in freeze-drying transportation, drying solid materials, drying and other directions, can solve the problems of unfreeze-dried process comparison, low production efficiency, high energy consumption, and shorten the freeze-dried process. Dry cycle, improve production efficiency, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
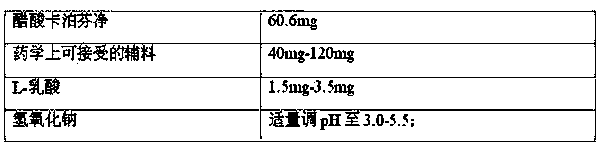
[0047] prescription:
[0048]
[0049] Preparation process: Weigh 90% of the total amount of water for injection and cool to room temperature, add L-lactic acid, stir well, adjust the pH value to 3.0-5.5 with sodium hydroxide solution; add sucrose and mannitol, stir to dissolve, add medicinal Charcoal, stirred and adsorbed, decarbonized and filtered. Cool the filtrate to below 15°C, add the prescribed amount of caspofungin acetate, stir to dissolve, and add water for injection to the full amount. Sterilization and filtration, filling, half-stoppering, putting into the box of a freeze dryer, freeze-drying, corking out of the box, and capping to obtain composition 1.
[0050] Freeze-drying process: cool down to -40°C to freeze, heat up to about -20°C for 20 minutes, continue to cool down to -40°C to freeze solid; vacuumize to 10Pa, heat up to -10°C for sublimation and drying; continue to heat up to 20°C to complete analysis dry.
Embodiment 2
[0052] prescription:
[0053]
[0054]
[0055] Preparation process: Weigh 90% of the total amount of water for injection and cool to room temperature, add L-lactic acid, stir well, adjust the pH value to 3.0-5.5 with sodium hydroxide solution; add sucrose and mannitol, stir to dissolve, add medicinal Charcoal, stirred and adsorbed, decarbonized and filtered. Cool the filtrate to below 15°C, add the prescribed amount of caspofungin acetate, stir to dissolve, and add water for injection to the full amount. Sterilization and filtration, filling, half-stoppering, putting into the box of a freeze dryer, freeze-drying, corking out of the box, and capping to obtain composition 2.
[0056] Freeze-drying process: cool down to -35°C for freezing, heat up to about -20°C for 30 minutes, continue to cool down to -35°C for freezing; vacuumize to 15Pa, heat up to -15°C for sublimation and drying; continue to heat up to 20°C to complete analysis dry.
Embodiment 3
[0058] prescription:
[0059]
[0060] Preparation process: Weigh 90% of the total amount of water for injection and cool to room temperature, add L-lactic acid, stir well, adjust the pH value to 3.0-5.5 with sodium hydroxide solution; add sucrose and mannitol, stir to dissolve, add medicinal Charcoal, stirred and adsorbed, decarbonized and filtered. Cool the filtrate to below 15°C, add the prescribed amount of caspofungin acetate, stir to dissolve, and add water for injection to the full amount. Sterilization and filtration, filling, half-stoppering, putting into the box of a freeze dryer, freeze-drying, corking out of the box, and capping to obtain composition 3.
[0061] Freeze-drying process: cool down to -32°C for freezing, heat up to about -20°C for 60 minutes, continue to cool down to -32°C for freezing; vacuumize to 20Pa, heat up to -5°C for sublimation and drying; continue to heat up to 20°C to complete analysis dry.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com