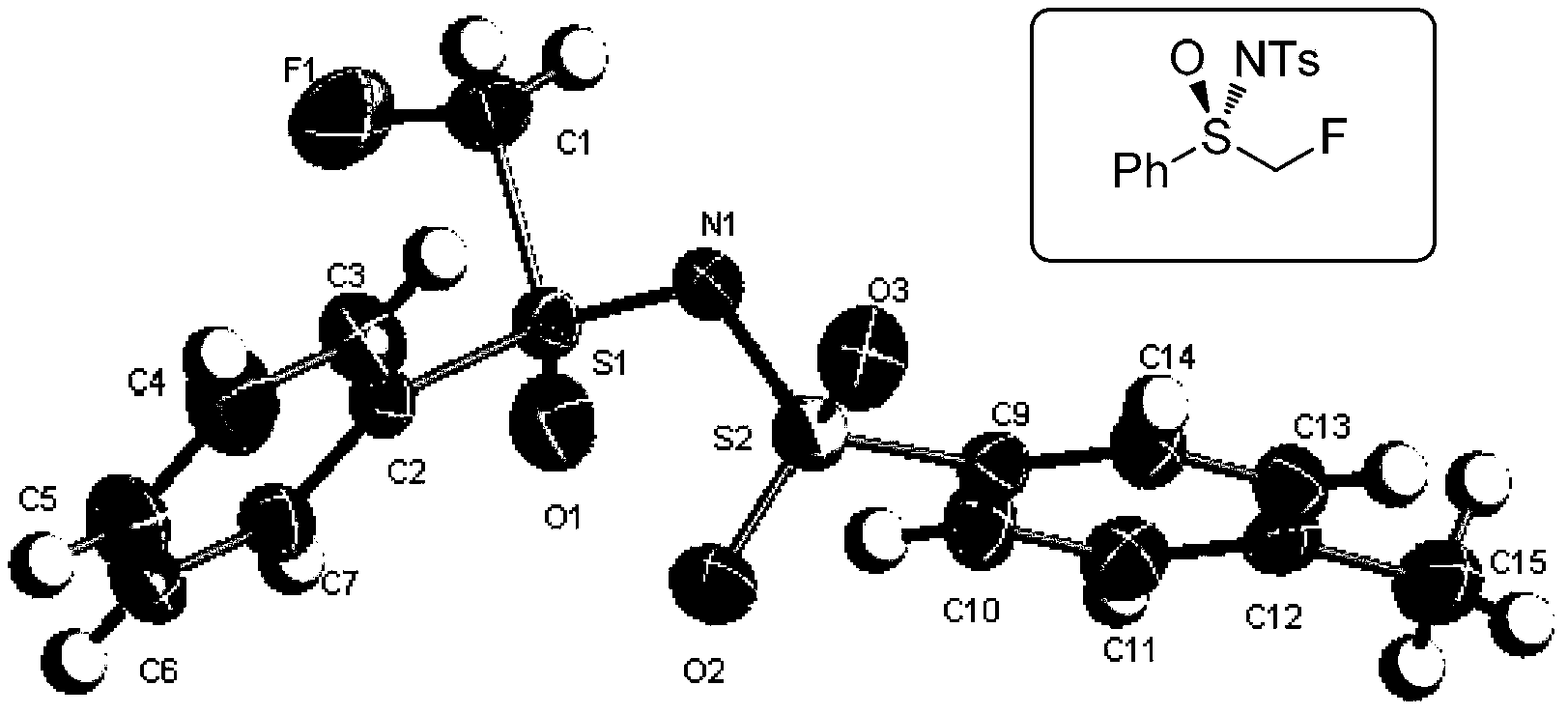
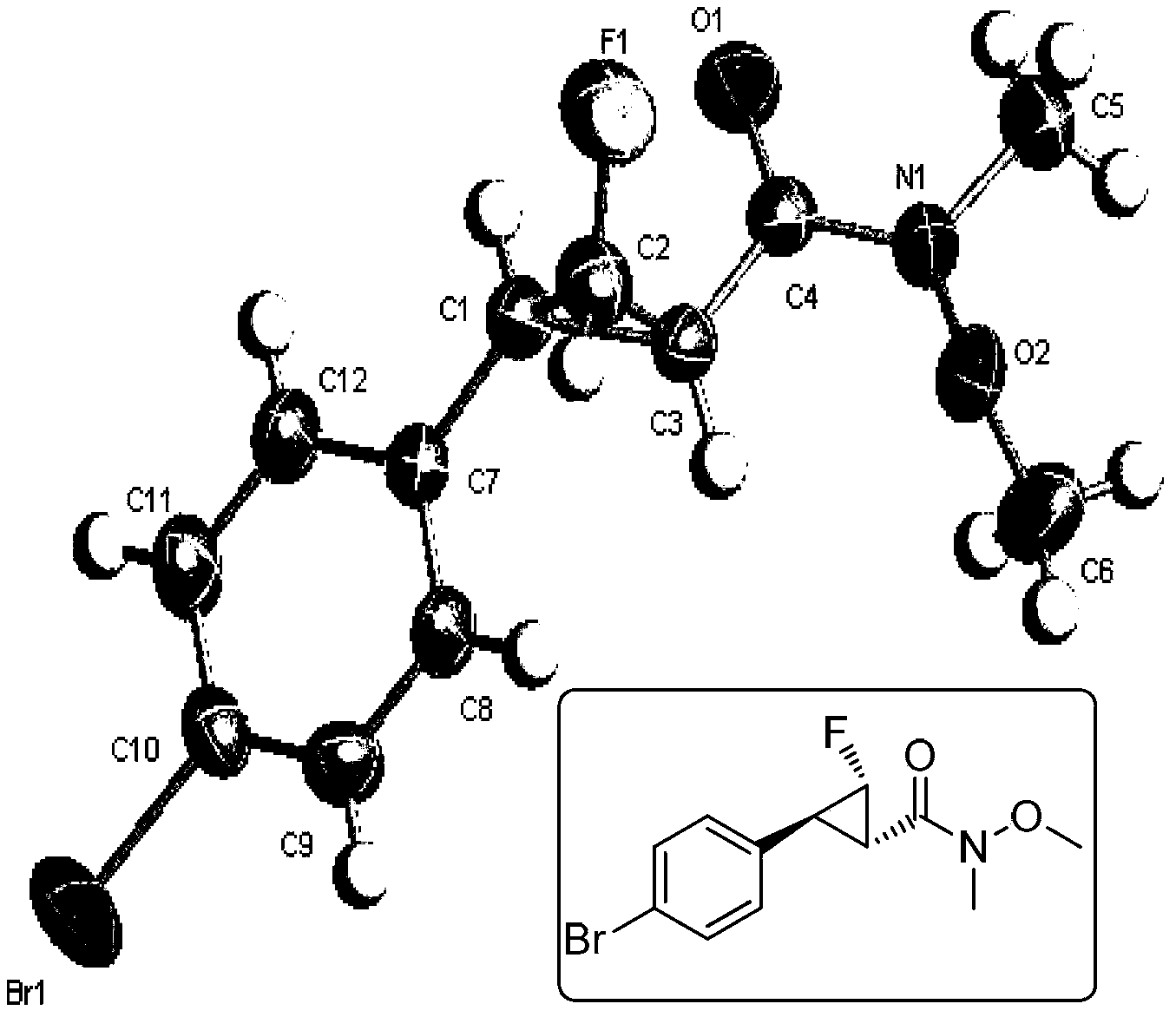
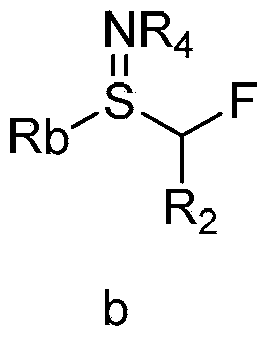
Cyclopropane derivatives, and preparation method and application thereof
An alkyl and reaction technology, applied in the field of cyclopropane derivatives and their preparation, can solve the problem of low stereoselectivity, no high stereoselectivity type synthesis of optically pure sulfoximine-substituted cyclopropane compounds, poor substrate universality, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0119] The preparation method of the compound of formula I provided by the invention comprises steps:
[0120] (a3) reacting the compound of formula b with an oxidation reagent to obtain the compound of formula II;
[0121]
[0122] (b) reacting a compound of formula III with a compound of formula II in an organic solvent and in the presence of a base to form the compound of formula I,
[0123]
[0124] In various forms, R 1 , R 2 , Ra, Rb, R 4 is defined as above;
[0125] In another preferred embodiment, in the step (b), the compound of formula III and the compound of formula II are reacted at -78°C to 60°C (preferably, -78°C to 25°C) for 0.5-72 hours (preferably 0.5-5 hours), to obtain the compound of formula I.
[0126] In another preference, in the step (b), the organic solvent is selected from: tetrahydrofuran THF, toluene PhCH 3 , dichloromethane CH 2 Cl 2 , Hexamethyl triamine phosphate HMPA, ethylene glycol dimethyl ether DME, ether Et 2 O, dimethylfo...
Embodiment 1
[0219] Example 1 (1S, 2S, 3S)-2-phenyl-3-fluoro-N-methoxy-N-methyl-cyclopropylcarbonamide (1S, 2S, 3S)-2-phenyl-3- Preparation of fluoro-N-methoxy-N-methyl-cyclopropanecarboxamide
[0220]
[0221] N 2 Under protection, [(R)-N-p-toluenesulfonyl] monofluoromethylphenyl sulfoximine (196mg, 0.6mmol), nitrogen-methyl-nitrogen-methoxy Cinnamamide (76mg, 0.4mmol) and 4mL of dry THF were cooled to -78°C, and LHMDS (lithium hexamethylsilylamide 0.52mmol) was slowly added dropwise, stirred for 20min, the cooling bath was removed, and the temperature was naturally raised to room temperature at 25 Stir at ℃ for 3 h, add 2 mL of water to quench the reaction, extract with anhydrous ether (15 mL×3), combine the organic phases, wash with saturated brine (5 mL), and dry over anhydrous magnesium sulfate. The solvent was removed by rotary evaporation under reduced pressure, and the product (80 mg, yield 90%) was obtained by flash column chromatography (5:1).
[0222] colorless liquid. Op...
Embodiment 2
[0224] Example 2 (1S, 2S, 3S)-2-(3-chlorophenyl)-3-fluoro-N-methoxy-N-methyl-cyclopropylcarbonamide (1S, 2S, 3S)-2 - Preparation of (3-chlorophenyl)-3-fluoro-N-methoxy-N-methyl-cyclopropanecarboxamide
[0225] N 2 Under protection, [(R)-N-p-toluenesulfonyl] monofluoromethylphenyl sulfoximine (196mg, 0.6mmol) was added to a 25mL Schlenk reaction tube, (90mg, 0.4mmol) and 4mL dry THF, cooled to -78°C, slowly added LHMDS (0.52mmol) dropwise, stirred for 20min, removed the cooling bath, naturally raised to room temperature 25°C and stirred for 3h, added 5mL of water to quench the reaction, Extract with anhydrous ether (15 mL×3), combine the organic phases, wash with saturated brine (5 mL), and dry over anhydrous magnesium sulfate. The solvent was removed by rotary evaporation under reduced pressure, and the product was obtained by flash column chromatography (5:1) (91 mg, yield 88%).
[0226] White solid. Melting point. 51–52°C. [α] D 28 =+110.0°(c=1.0,CH 2 Cl 2 ). The ena...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com