A purifying method of a novel antibiotic compound
A purification method and antibiotic technology, applied in the field of medicine, can solve the problems of low volatility, easy residue and high product impurity content, and achieve the effects of reducing the generation of degradation impurities, reducing the impurity content of the product and improving the product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
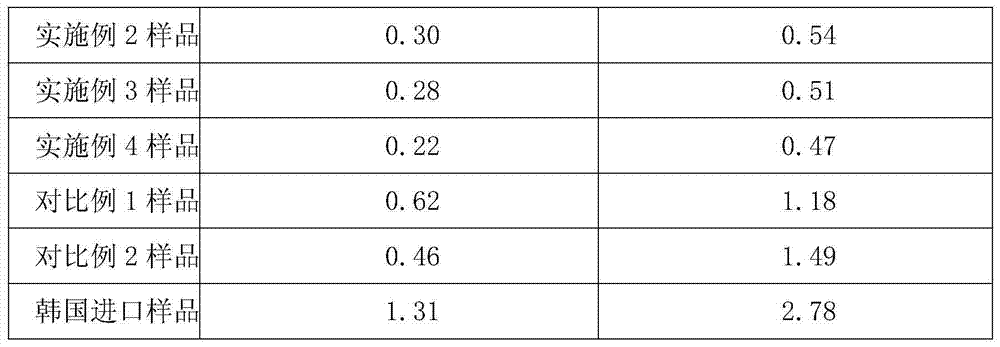
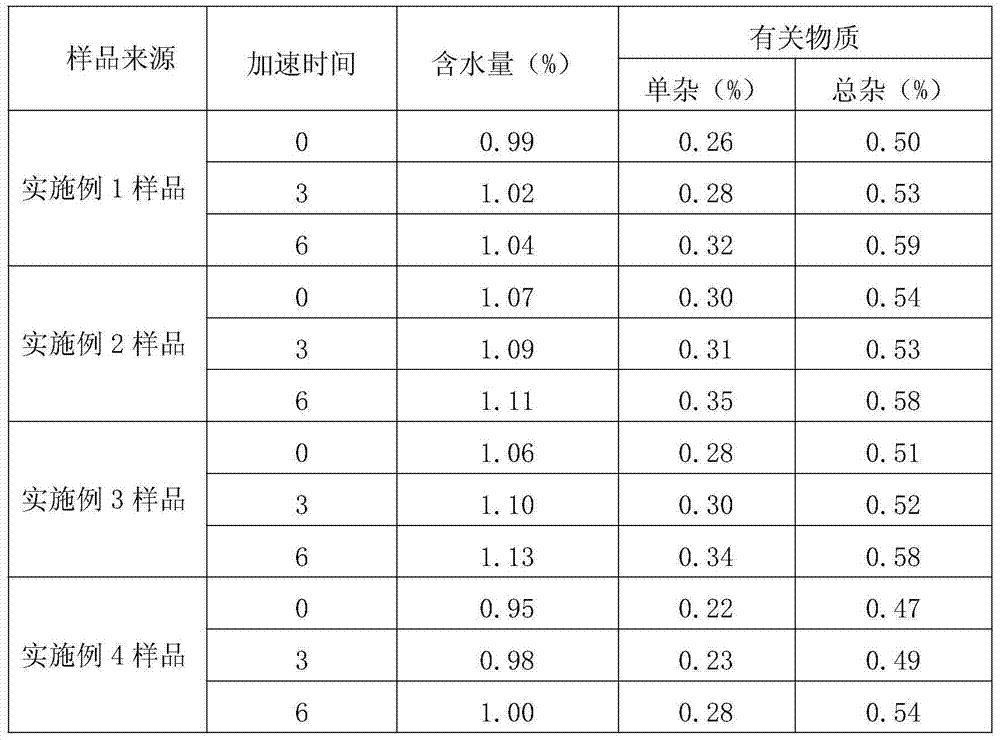
Embodiment 1
[0032] A kind of refining method of novel antibiotic compound cefazedone sodium, the steps are as follows:
[0033] (1) Salt formation: Aseptically treat the reaction kettle, filter, and utensils used, and add 1096.9g (2mol) cefazinone, 398.9g (2.4mol) sodium isooctanoate and 6.58L anhydrous methanol to a 50L reaction kettle , stir to dissolve completely, and control the temperature at 15-35°C;
[0034] (2) Decolorization: Add 11g of activated carbon into the reaction solution, stir and absorb for 30 minutes, filter the titanium rod for decarbonization, and pass the filtrate through a 0.22 μm microporous membrane for further decarbonization and sterilization, and keep the temperature of the filtrate at 10-30°C;
[0035] (3) Crystallization: The filtrate is transferred to a 50L crystallization tank in a local clean area of 10,000 grades, and a mixed solvent of 5.86L acetone and 2.93L ethyl acetate is added to the filtered filtrate, and the temperature is controlled at 15-20°C...
Embodiment 2
[0038] A kind of refining method of novel antibiotic compound cefazedone sodium, the steps are as follows:
[0039] (1) Salt formation: Aseptically treat the reaction kettle, filter and utensils used, and add 1096.9g (2mol) cefazinone, 465.33g (2.8mol) sodium isooctanoate and 8.78L anhydrous methanol to a 50L reaction kettle , stir to dissolve completely, and control the temperature at 15-35°C;
[0040] (2) Decolorization: Add 22g of activated carbon into the reaction solution, stir and absorb for 30 minutes, filter the titanium rod for decarbonization, and pass the filtrate through a 0.22μm microporous membrane for further decarbonization and sterilization, and keep the temperature of the filtrate at 10-30°C;
[0041] (3) Crystallization: The filtrate is transferred to a 50L crystallization tank in a local clean area of 10,000 grades, and a mixed solvent of 7.32L acetone and 3.66L ethyl acetate is added to the filtered filtrate, and the temperature is controlled at 15-20°C....
Embodiment 3
[0044] A kind of refining method of novel antibiotic compound cefazedone sodium, the steps are as follows:
[0045](1) Salt formation: Aseptically treat the reaction kettle, filter and utensils used, and add 1096.9g (2mol) cefazedone, 531.8g (3.2mol) sodium acetate and 10.97L anhydrous methanol into the 50L reaction kettle , stir to dissolve completely, and control the temperature at 15-35°C;
[0046] (2) Decolorization: Add 32.9g of activated carbon into the reaction solution, stir and adsorb for 30 minutes, filter the titanium rod for decarbonization, and pass the filtrate through a 0.22μm microporous membrane for further decarbonization and sterilization, and keep the temperature of the filtrate at 10-30°C;
[0047] (3) Crystallization: The filtrate is transferred to a 50L crystallization tank in a local clean area of 10,000 grades, and a mixed solvent of 8.78L acetone and 4.39L ethyl acetate is added to the filtered filtrate, and the temperature is controlled at 15-20°C....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com