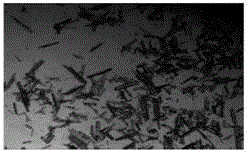
A crystallization method of erythromycin ethylsuccinate with controllable crystal habit and particle size
A technology of erythromycin ethylsuccinate and crystallization, which is applied in the field of preparation of pharmaceutical compounds, can solve the problems of small product particle size, inability to meet the quality of raw materials and preparations, and easy coalescence of flaky crystals.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Add 100L of erythromycin ethylsuccinate tetrahydrofuran reaction solution to the 500L crystallization kettle, control the stirring rate at 250rpm, control the temperature of the reaction solution at 40°C, add the initial amount of 100L of dissolving agent water through multiple feeding ports of the crystallizer coil, and A two-step program cooling method is adopted to precipitate crystals, that is, in the first step, the temperature of the reaction solution is lowered from 40°C to 35°C while continuing to add water to dissolve, and the amount of water added to the dissolution process is 100L, and the dissolution is completed; the second step is to react The liquid temperature was lowered from 35°C to 20°C. The time of the whole crystallization process of erythromycin ethylsuccinate was controlled at 1.0 hour. Filter and dry under reduced pressure to obtain 20.0 kg of erythromycin ethylsuccinate crystals. The obtained crystals are long rod-shaped crystals with a main par...
Embodiment 2
[0092] Add 100L erythromycin ethylsuccinate tetrahydrofuran reaction liquid in the 500L crystallization kettle, control the stirring rate at 300rpm, control the temperature of the reaction liquid at 40°C, add the initial amount of 110L of dissolving agent water through multiple feeding ports of the crystallizer coil, and A two-step step-by-step program cooling method is used to precipitate crystals, that is, the first step continues to add water to dissolve the temperature of the reaction solution from 40 ° C to 35 ° C, the amount of water added during the dissolution process is 200 L, and the dissolution is completed; the second step will be The temperature of the reaction solution was lowered from 35°C to 20°C. The time of the whole crystallization process of erythromycin ethylsuccinate was controlled within 2 hours. Filtration and drying under reduced pressure gave 21 kg of erythromycin ethylsuccinate crystals. The obtained crystals were long rod-shaped crystals with a main...
Embodiment 3
[0094] Add 100L erythromycin ethylsuccinate tetrahydrofuran reaction liquid in the 500L crystallization kettle, control the stirring rate at 400rpm, control the temperature of the reaction liquid at 40°C, add the initial amount of 100L of dissolving agent water through multiple feeding ports of the crystallizer coil, and A two-step step-by-step program cooling method is used to precipitate crystals, that is, in the first step, the temperature of the reaction solution is lowered from 40°C to 35°C while continuing to add water for dissolution, and the amount of water added during the dissolution process is 300L, and the dissolution is completed; The temperature of the reaction solution was lowered from 35°C to 20°C. The time of the whole crystallization process of erythromycin ethylsuccinate was controlled within 1 hour. Filter and dry under normal pressure to obtain 22.93 kg of erythromycin ethylsuccinate crystals. The obtained crystals are long rod-shaped crystals with a main ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com