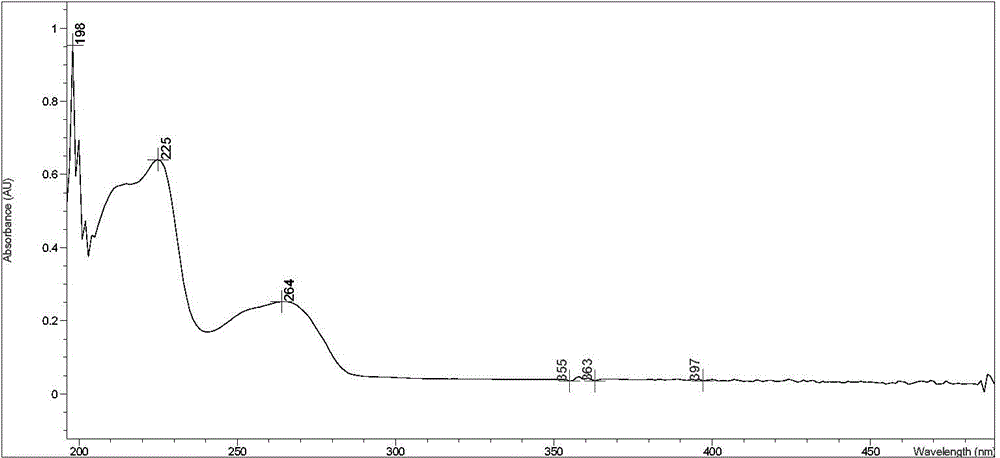
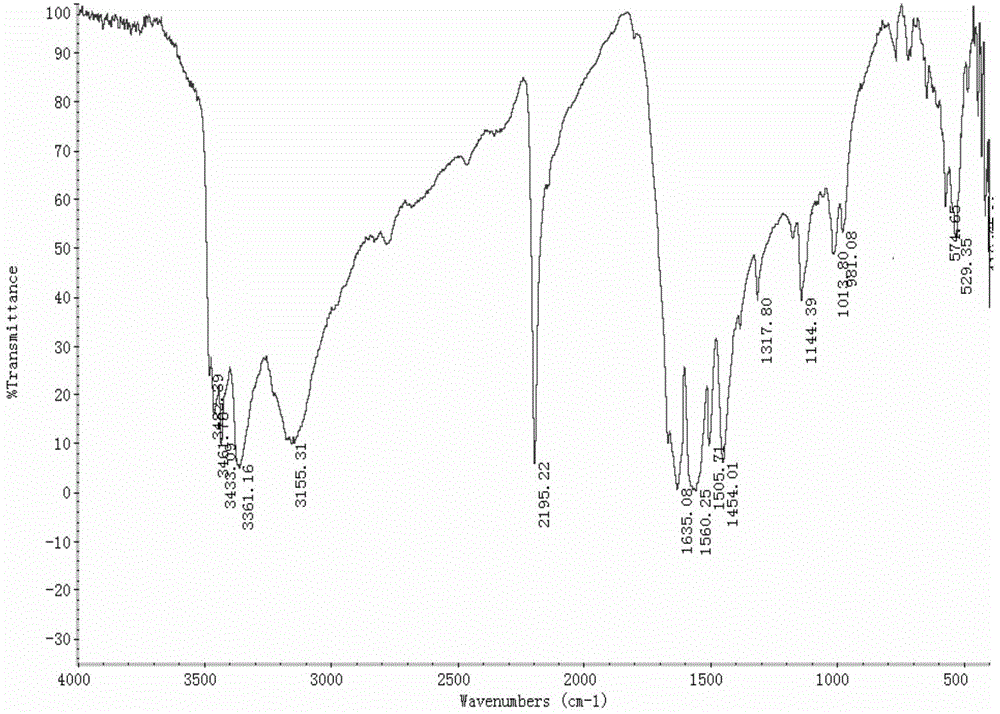
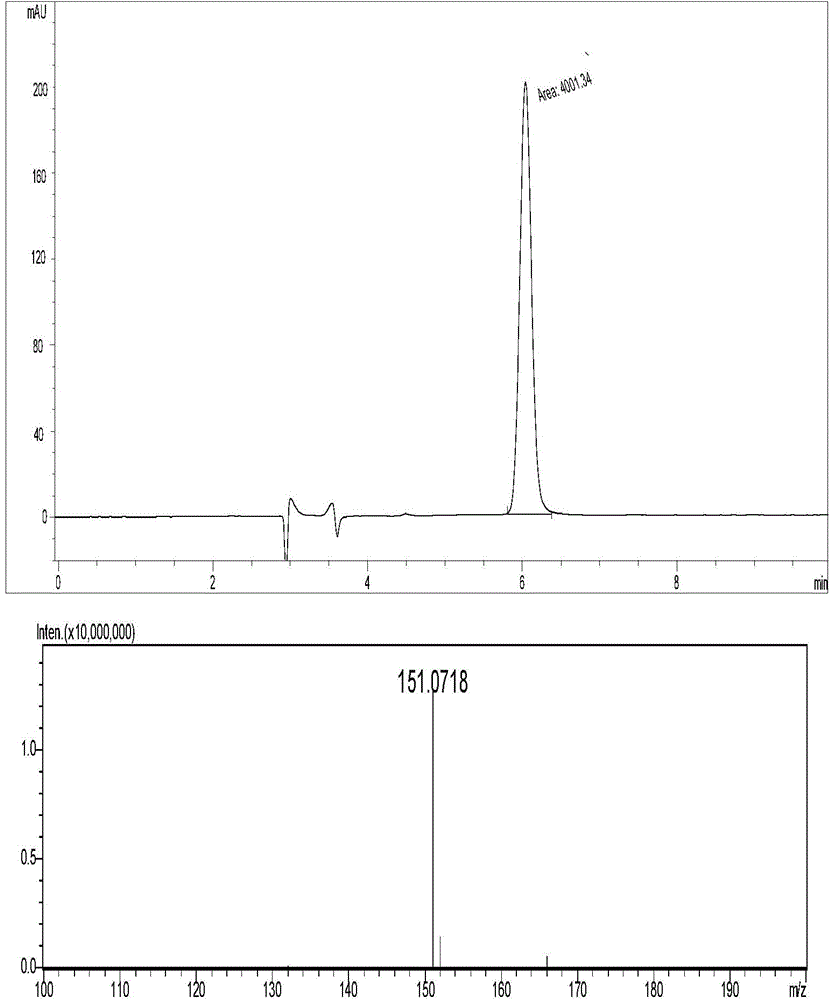
Chemical synthetic method of 2,4,6-triamido-5-cyano pyrimidine
A technology of cyanopyrimidine and synthesis method, which is applied in the field of chemical synthesis of 2,4,6-triamino-5-cyanopyrimidine, can solve the problems of many route steps, difficult to obtain, not given and the like, and achieves reaction conditions The effect of simplicity, high reaction yield and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Dissolve 20 g of sodium dicyandiamide in 70 mL of dimethyl sulfoxide solution, stir and dissolve at 120 ° C, and add dropwise 20 mL of dimethyl sulfoxide solution dissolving 15 g of malononitrile in the reaction flask, where The reaction was carried out at high temperature for 5 h, and the reaction process was monitored by TLC. After completion of the reaction, cool to room temperature, wash the slurry with 50 mL of sec-butanol for 30 min, until a light yellow solid is formed, filter with suction, and dry the resulting product in a vacuum oven at 50°C for 5 h to obtain 1-amino-1-cyanoamino-2 ,2-Dicyanoethene sodium salt 26.2g, yield 75%.
[0033] (2) Dissolve 20g of 1-amino-1-cyanoamino-2,2-dicyanoethene sodium salt in 300mL of sec-butanol solution, seal it tightly, introduce hydrogen chloride gas into the mixture, and keep it saturated for 2 hours. The reaction was stirred at ℃ for 18 h, and the reaction process was monitored by TLC. After the reaction was comple...
Embodiment 2
[0036] (1) Dissolve 20g of sodium dicyandiamide in 130mL of N,N-dimethylformamide, stir and dissolve at 160°C, add dropwise 20mL of N,N-dimethylformamide that dissolves 28g of malononitrile into the reaction bottle Formamide solution was reacted at this temperature for 3 h, and the reaction process was monitored by TLC. After completion of the reaction, cool to room temperature, wash the slurry with 50 mL of sec-butanol for 30 min, until a light yellow solid is formed, filter with suction, and dry the resulting product in a vacuum oven at 50°C for 5 h to obtain 1-amino-1-cyanoamino-2 ,2-Dicyanoethene sodium salt 34.5g, yield 99%.
[0037] (2) Dissolve 20g of 1-amino-1-cyanoamino-2,2-dicyanoethene sodium salt in 150mL of sec-butanol solution, seal it tightly, introduce hydrogen chloride gas into the mixture, keep it saturated for 2 hours, 0~5℃ The reaction was stirred for 20 h, and the reaction process was monitored by TLC. After the reaction was completed, the slurry was sucti...
Embodiment 3
[0040] (1) Dissolve 20g of sodium dicyandiamide in 200mL of acetonitrile solution, stir and dissolve at 140°C, add dropwise 10mL of acetonitrile solution in which 15g of malononitrile is dissolved in the reaction flask, react at this temperature for 2h, and monitor the reaction process by TLC . After completion of the reaction, cool to room temperature, wash the slurry with 50 mL of sec-butanol for 30 min, until a light yellow solid is formed, filter with suction, and dry the resulting product in a vacuum oven at 50°C for 5 h to obtain 1-amino-1-cyanoamino-2 ,2-Dicyanoethene sodium salt 24.8g, yield 71%.
[0041] (2) Dissolve 20g of 1-amino-1-cyanoamino-2,2-dicyanoethylene sodium salt in 100mL of methanol solution, seal it, add 50mL of 38% concentrated hydrochloric acid dropwise, stir and react at room temperature for 24h, and monitor the reaction process by TLC . After the reaction was completed, the slurry was filtered with suction, the filter cake was washed with a large ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com