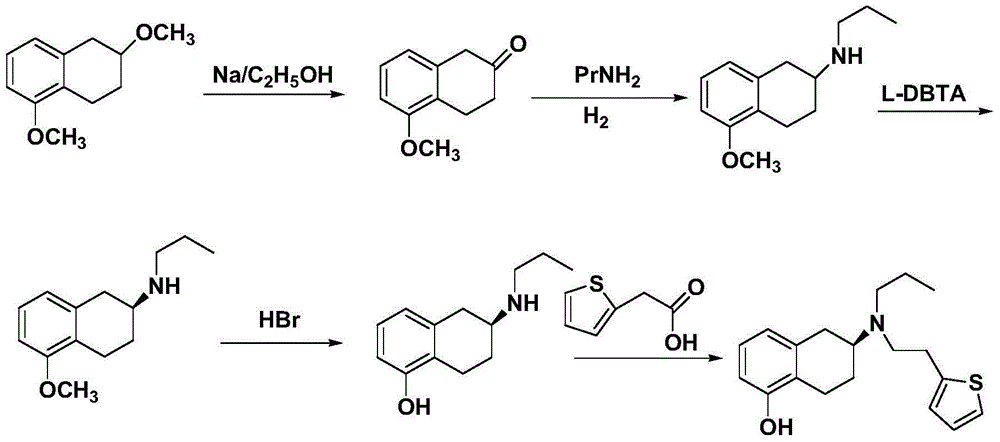
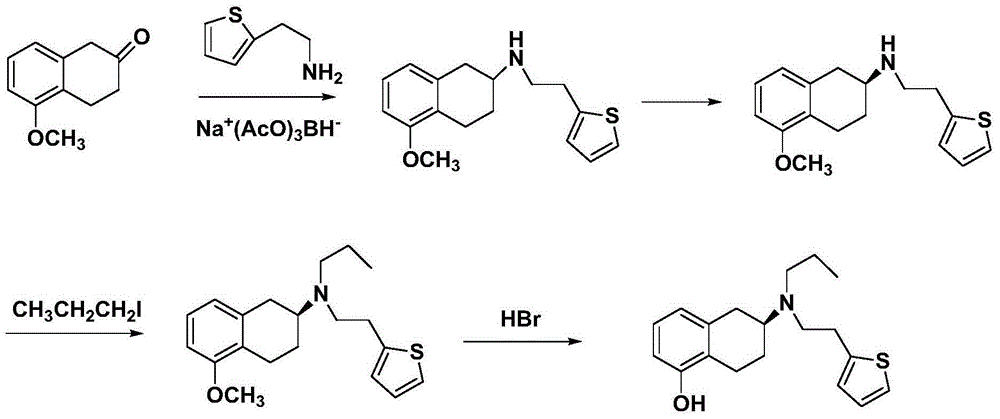
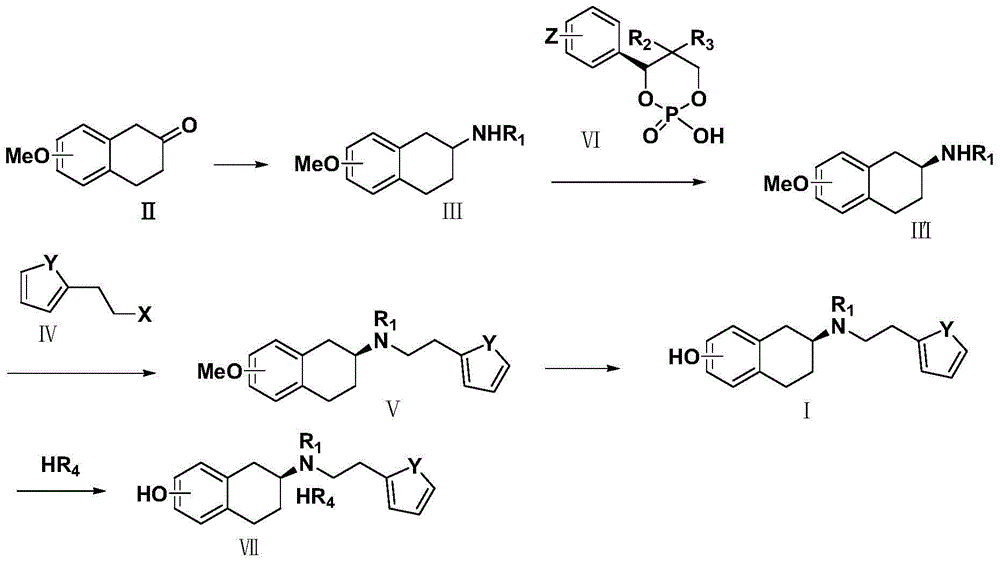
Preparation method of rotigotine
A technology of thiophene and solvent, applied in the new process field of rotigotine preparation, can solve the problems of increasing process steps and reducing yield, and achieve the effect of increasing yield and shortening synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0035] For a better description of the present invention, the specific implementation is as follows:
example 1
[0037] (1) Take 3.52g (0.02mol) of 5-methoxy-2-tetralone, dissolve it in 20mL of dichloromethane, add 2 drops of glacial acetic acid dropwise, heat to 50°C for reflux reaction, and then slowly add 3.3 mL (0.04mol) n-propylamine, reacted for 5 hours. After the reaction was completed, filter and wash with 50 mL of isopropanol for 2 to 3 times to obtain 3.47 g of khaki solid compound imine (I).
[0038] (2) Dissolve 3.47g of compound imine (I), 5g of Hans ester 1,4-dihydropyridine (HEH) in toluene, add chiral 3,3′-(trifluoromethyl)phenylbis Naphthol phosphate 0.2g, Molecular sieve 3g, reflux at 60°C for 3 hours, filter, remove the solvent under pressure, the crude product is separated through a silica gel column, using chloroform and methanol as developing solvents to obtain a yellow solid, which is dried to obtain 2.10 g of chiral compound (II).
[0039] C 14 h 21 NO, 1 HNMR (400MHZ, DMSO): -CH 3 (δ=0.998,m,3H),-CH 2 (δ=1.718,m,3H),-NH(δ=2.294,m,1H),-CH 2 (δ=2.909,m,4H)...
example 2
[0044] (1) Take 1.056g (0.006mol) of 5-methoxy-2-tetralone, dissolve it in 5mL of dichloromethane, add 0.2mL of glacial acetic acid dropwise, heat to 70°C for reflux reaction, and then slowly add 1mL dropwise (0.012mol) n-propylamine, reacted for 5 hours. After the reaction was completed, filter and wash with 6 mL of isopropanol for 2 to 3 times to obtain 1.04 g of khaki solid compound imine (I).
[0045] (2) Dissolve 1.04g of compound imine (I), 1.2g of Hans ester 1,4-dihydropyridine (HEH) in toluene, add chiral 3,3'-(trisilylmethyl)phenyl Binaphthyl Phosphate 0.05g, Molecular sieve 1g, reflux at 40°C for 3.5 hours, filter, remove the solvent under pressure, the crude product was separated through a silica gel column, using chloroform and methanol as developing solvents to obtain a yellow solid, which was dried to obtain 0.63g of chiral compound (II). C 14 h 21 NO, 1 HNMR (400MHZ, DMSO): -CH 3 (δ=0.998,m,3H),-CH 2 (δ=1.718,m,3H),-NH(δ=2.294,m,1H),-CH 2 (δ=2.909,m,4H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com