Preparation method of lubiprostone
A technology of lubiprostone and compounds, applied in the field of drug synthesis, can solve the problems of unsuitability for industrial production, difficulty in purification, and low product purity, and achieve the effects of strong process operability, high sample purity, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: 7-[(1R,2R,3R,5S)-2-(4,4-difluoro-3-hydroxyoctyl)-5-hydroxy-3-(2-tetrahydropyranyloxy) Synthesis of cyclopentyl]heptanoic acid (III)
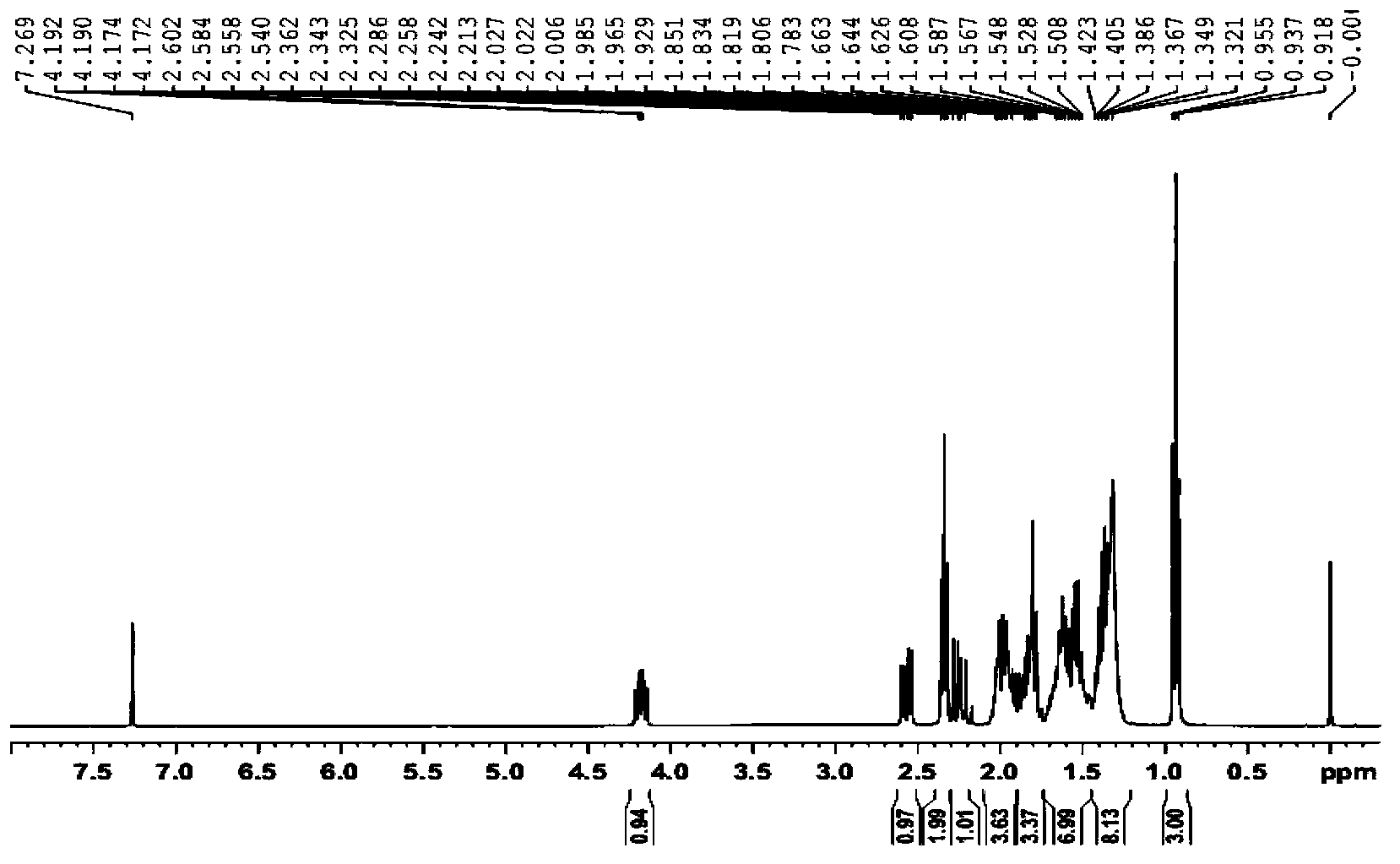
[0027] Dissolve 140g of compound (IV) in 280ml of ethyl acetate, add 10%Pd / C (50%H 2 O) 50g, replace hydrogen, react at room temperature and normal pressure for 2 hours, filter, and concentrate the filtrate under reduced pressure to obtain 7-[(1R,2R,3R,5S)-2-(4,4-difluoro-3-hydroxyoctyl )-5-Hydroxy-3-(2-tetrahydropyranyloxy)cyclopentyl]heptanoic acid (III) 140g, white solid, directly put into the next step reaction. 1 H NMR (200MHz, CDCl 3 ):δ(ppm):4.71-4.58(1H,m),4.18-3.96(2H,m),3.96-3.60(2H,m),3.60-3.42(1H,m),2.35(2H,t,J =7.6Hz), 2.13-1.17(30H,m), 0.93(3H,t,J=7.2Hz).
Embodiment 2
[0028] Example 2: 7-[(1R,2R,3R,5S)-2-(4,4-difluoro-3-oxooctyl)-5-oxo-3-(2-tetrahydropyranyloxy base) cyclopentyl] heptanoic acid (II) synthesis
[0029] Dissolve 120g of compound (III) in 2400ml of dichloromethane, add 160g of DMP, react at room temperature for 14 hours, add saturated aqueous sodium sulfite to quench the excess DMP, and after it is basically clear, add 300ml of saturated aqueous sodium bicarbonate, and stir for 20 minutes. Add ethyl acetate for extraction, combine the organic phases, dry over anhydrous sodium sulfate, filter, concentrate, and use petroleum ether / ethyl acetate column chromatography to obtain 7-[(1R,2R,3R,5S)-2-(4, 4-Difluoro-3-oxooctyl)-5-oxo-3-(2-tetrahydropyranyloxy)cyclopentyl]heptanoic acid (II) 100g, light yellow oil, yield: 84 %. 1 HNMR (200MHz, CDCl 3 ):δ(ppm):4.71-4.58(1H,m),4.18-3.96(2H,m),3.96-3.60(2H,m),3.60-3.42(1H,m),2.35(2H,t,J =7.5Hz), 2.13-1.17(30H,m), 0.93(3H,t,J=7.1Hz).
Embodiment 3
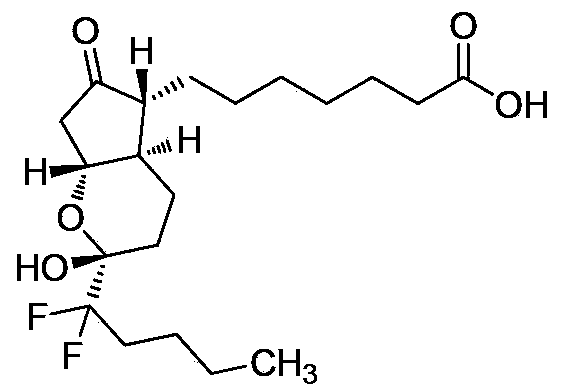
[0030] Example 3: (-)-7-[(2R,4aR,5R,7aR)-2-(1,1-difluoropentyl)-2-hydroxyl-6-oxooctahydrocyclopenta[b ]pyran-5-yl)heptanoic acid (I) synthesis
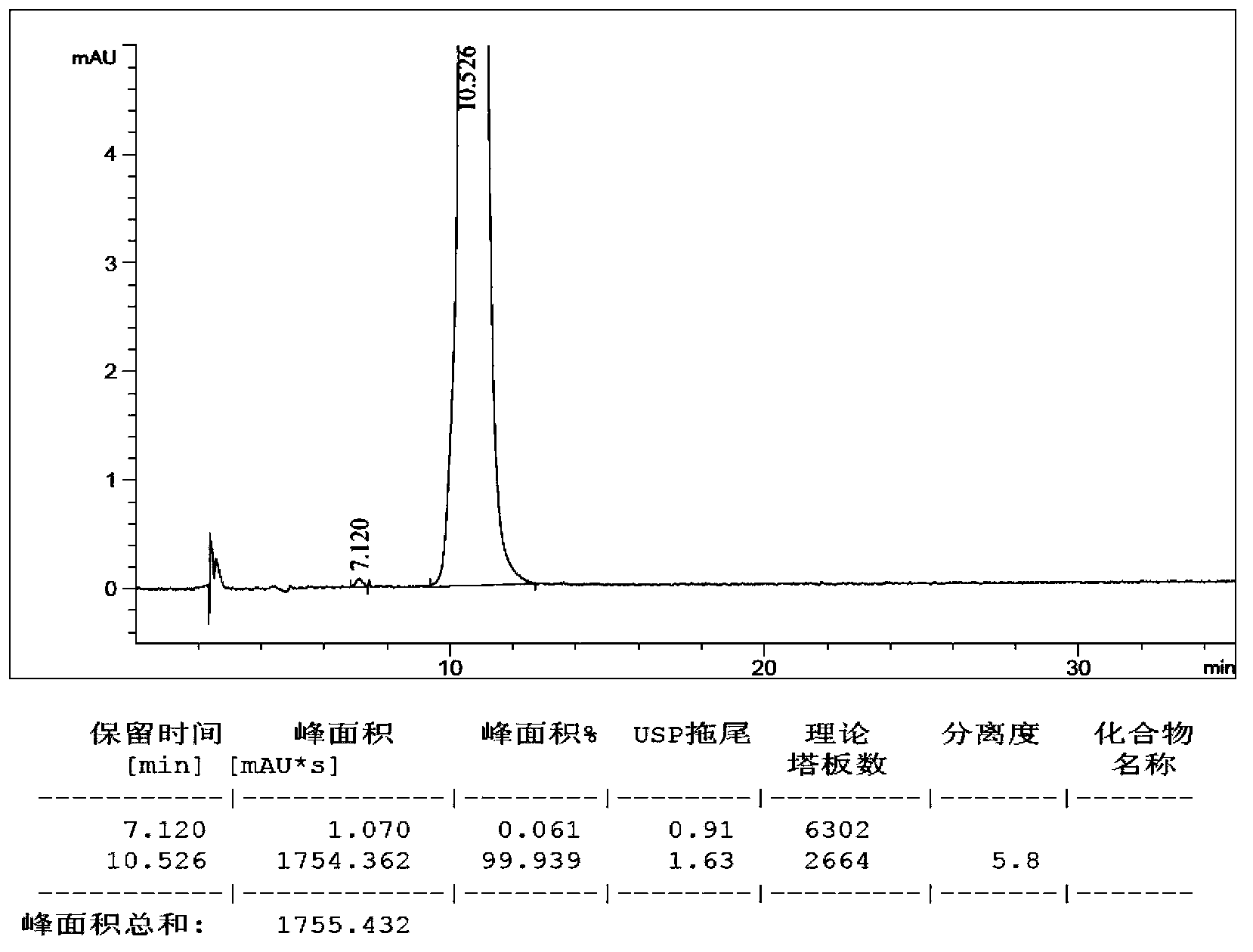
[0031] Cool 100g of compound (II) and 500ml of acetonitrile to 0-5°C, and add a mixed solution of acetonitrile / 85% phosphoric acid / water=100ml / 500ml / 100ml. After the addition, keep the reaction for 2 hours, dilute with 1000ml of water, extract three times with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, filter, concentrate, use petroleum ether / ethyl acetate column chromatography, get lubiprostone The crude product is about 81g. It was crystallized with ethyl acetate / n-hexane and dried to obtain 75.7 g of high-purity lubiprostone as a white solid, yield: 92%, HPLC: 99.94%.
[0032] (Preparation of starting materials): Compound (IV) used in the present invention: 7-[(1R,2R,3R,5S)-2-(4,4-difluoro-3-hydroxyoctyl)-5-hydroxyl -3-(2-tetrahydropyranyloxy)cyclopentyl]heptanoic acid was prepared by the follo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com