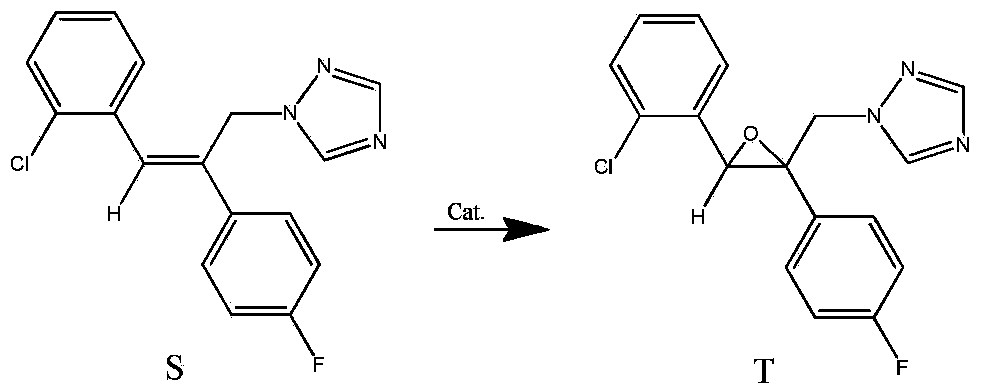
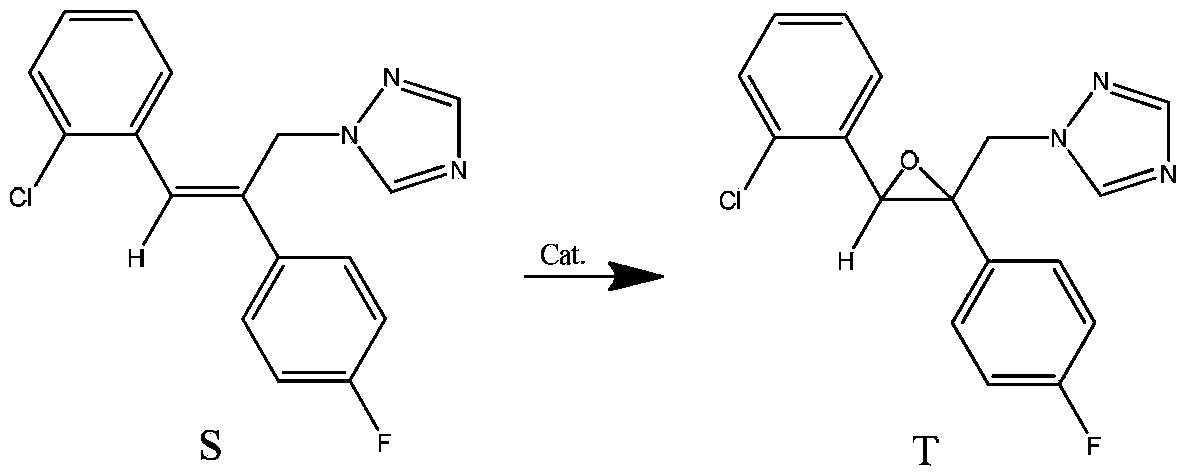
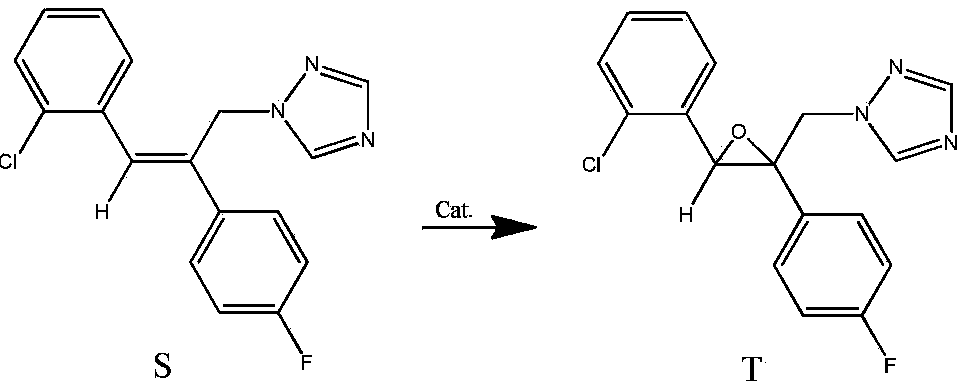
Method for synthesizing epoxiconazole by catalyzing air or oxygen epoxidation
A technology for synthesizing epoxiconazole and epoxidation, which is applied in the direction of organic chemistry, can solve the problems of increased post-processing difficulty, increased production cost, and poor selectivity of waste acid, and achieve high product yield, reduction of three wastes, and The effect of high conversion rate of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In a glass reactor, dissolve 0.03g (0.05mmol) tris(dibenzoylmethane)iron, 0.78g (2.5mmol) triazolene, 1.08g (14.9mmol) isobutyraldehyde in 20mL 1,2-dichloroethyl In alkanes, the temperature was raised to 50° C. with stirring, and air at one atmosphere was maintained, and kept stirring at this temperature for 18 hours. Heating was stopped, the material was naturally cooled to room temperature, and sampling analysis showed that the conversion of triazole was 87.0%, the selectivity of epoxiconazole was 78.1%, and the yield of epoxiconazole was 67.9%.
Embodiment 2
[0027] Use 0.15g (0.25mmol) tris(dibenzoylmethane)iron instead, other conditions are the same as in Example 1, stir at room temperature for 24 hours, heat up to 50°C and react at this temperature for 8 hours, stop heating, and let the material cool naturally to room temperature. Sampling analysis showed that the conversion rate of triazolene was 76.3%, the selectivity of epoxiconazole was 78.0%, and the yield of epoxiconazole was 59.5%.
Embodiment 3
[0029] In a glass reactor, dissolve 0.14g (0.28mmol) bis(dibenzoylmethane) cobalt, 0.78g (2.5mmol) triazolene, 1.08g (14.9mmol) isobutyraldehyde in 10mL 1,2-dichloroethyl In alkanes, keep one atmospheric pressure of air, stir at room temperature for 19 hours, be warmed up to 50 ° C and react at this temperature for 8 hours, stop heating, and the material is naturally cooled to room temperature. Sampling analysis showed that the conversion rate of triazolene was 71.0%, the selectivity of epoxiconazole was 77.0%, and the yield of epoxiconazole was 54.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com