Method for preparing human pregnancy-specific glycoprotein 9
A technology of protein and recombinant bacteria, applied in the field of genetic engineering, can solve the problem of no specific antibody
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1, the construction of human PSG9 genetic engineering strain
[0027] 1. Cloning of the full-length gene of human PSG9 by polymerase chain reaction
[0028] Using the upstream and downstream primer sequences, the full-length human PSG9 coding gene sequence was cloned using the pcDNA3.1-PSG9 plasmid vector as a template. The primer sequences are as follows:
[0029] Upstream primer sequence: 5'-CTA GCTAGC ACAGGCAGCAGAGACCATGGG-3', the underline represents the NheI restriction site;
[0030] Downstream primer sequence: 5'-CC AAGCTT TACAGTCTCAGAGTCAGTCATGA-3', the underline represents the Hind III restriction site, and the downstream stop codon was replaced with CAT.
[0031] It is also possible to artificially synthesize the gene shown in nucleotides 1-1278 in SEQ ID NO.1, and add Nhe I restriction sites and Hind III restriction sites upstream and downstream.
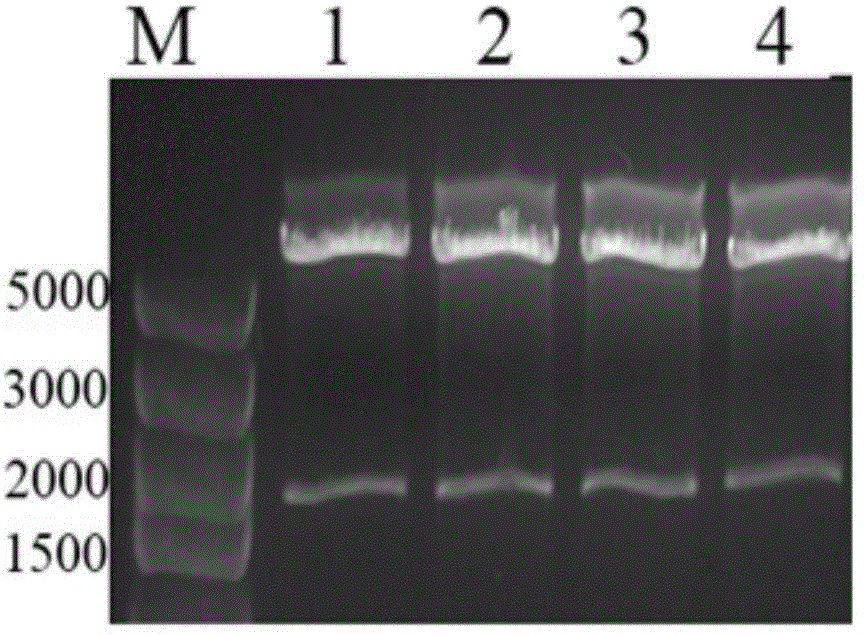
[0032] 2. Double digestion and ligation
[0033] The PCR amplified product was digested with ...
Embodiment 2
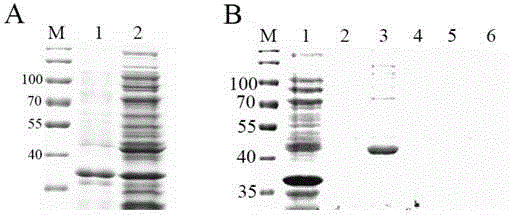
[0036] Embodiment 2, expression and purification of PSG9 protein
[0037] 1. Exploration of expression conditions of PSG9-6×His protein
[0038] When E.coli BL21(DE3)-PSG9-6×His was cultured until the OD value of the bacterial solution was 0.6, the target protein PSG9 was induced with the inducer Isopropylβ-D-1-Thiogalactopyranoside (IPTG) -6 x His expression. In order to obtain a high yield of PSG9-6×His protein, we optimized the concentration of IPTG and the induction temperature.
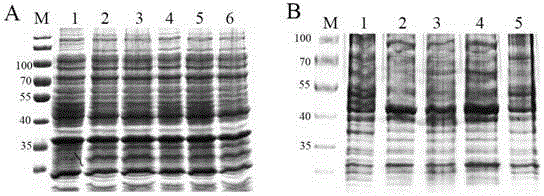
[0039] The expression of the target protein PSG9-6×His was induced by different concentrations (0 μM, 0.5 μM, 1 μM, 2 μM, 3 μM, 5 μM) of IPTG. The results showed that when the concentration of IPTG was 3 μM, the concentration of the target protein reached the maximum ( figure 2 A).
[0040] The expression of the target protein PSG9-6×His was induced by IPTG at different temperatures (20°C, 25°C, 30°C, 37°C), and the concentration of IPTG in the bacterial solution was 3 μM. The results showe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com