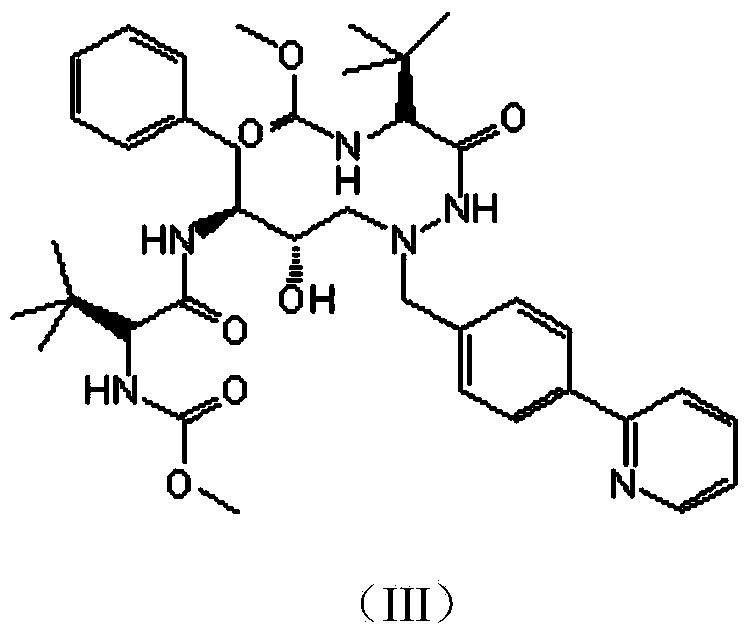
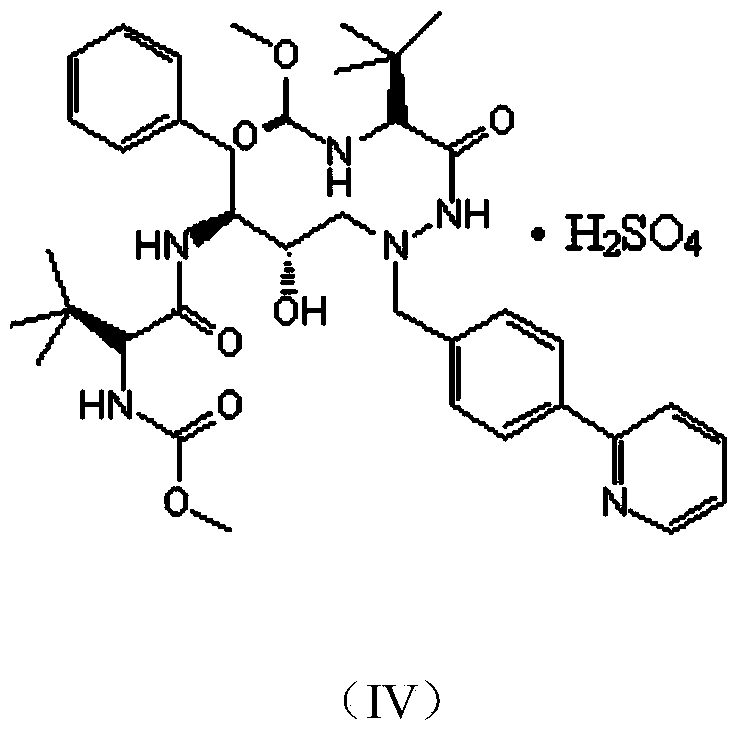
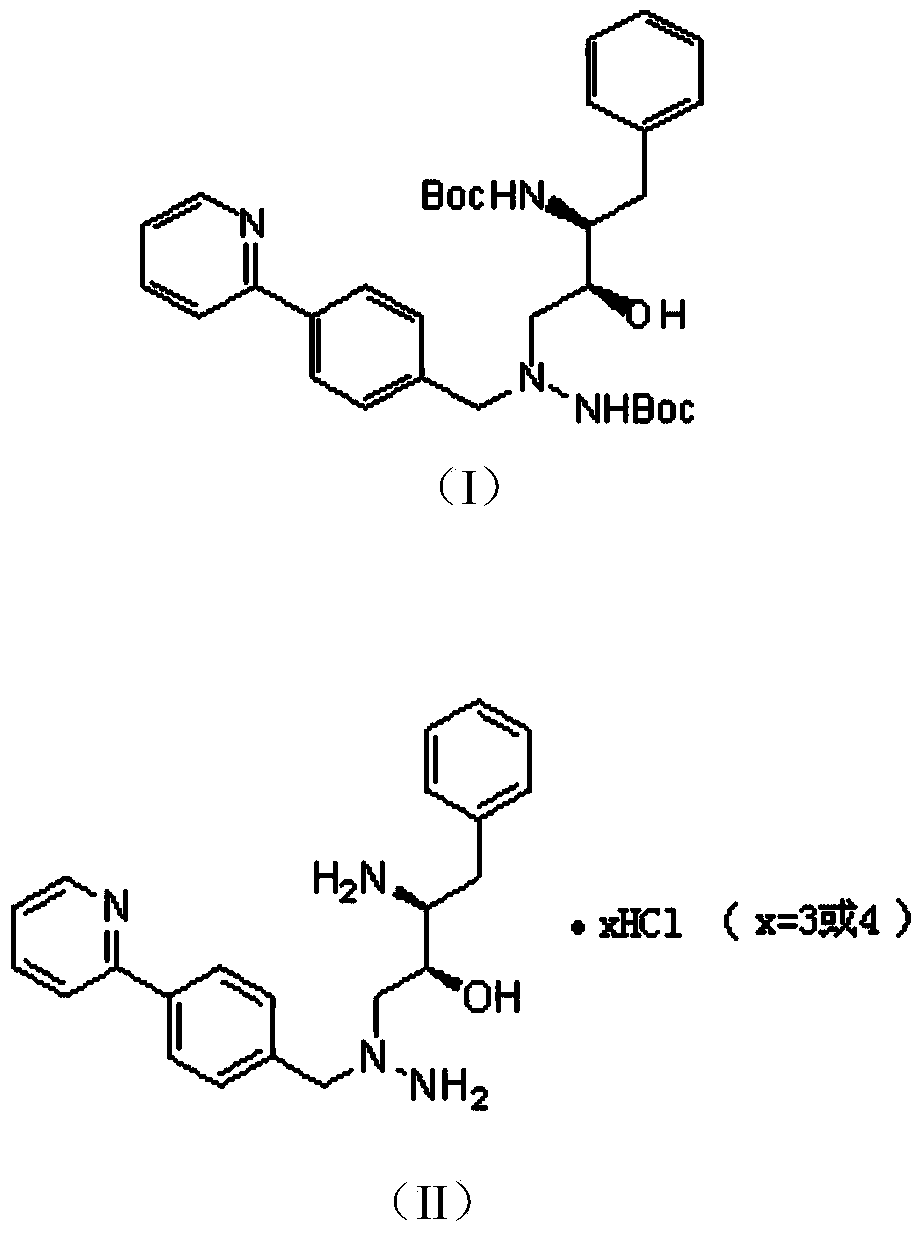
Preparation methods of Atazanavir and sulfate of Atazanavir
A technology of intermediates and raw materials, applied in the field of preparation of atazanavir and its sulfate, can solve the problems of multiple impurities, low yield, high product purification cost, etc., and achieve the goal of accelerating reaction rate, reducing production cost and reducing purification The effect of times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The synthesis of the intermediate shown in embodiment 1 formula (I)
[0049]
[0050] In a 500mL three-necked flask, add 50.0g of the intermediate represented by formula (a), 50.5g of the raw material represented by formula (b) and 250mL of tert-butanol, and heat the oil bath to 90°C. After reacting for 3 hours, solids gradually precipitated out. Stirring was continued for 18 hours, and a sample was taken for HPLC detection.
[0051] HPLC analysis method: chromatograph: Dionex v3000DAD
[0052] Column: Inertsil ODS-3
[0053] Chromatographic conditions: acetonitrile: phosphate buffer = 70: 30, where the phosphate buffer is K 2 HPO 3 -KH 2 PO 3 Buffer (0.02M K 2 HPO 3 and 0.02M KH 2 PO 3 )
[0054] Detection wavelength: UV210nm
[0055] Column temperature: 30°C
[0056] Flow rate: 1.5mL / min
[0057] The intermediate shown in formula (a) is detected to be less than 1.5% by HPLC, stop heating, cool down to 30°C, add 750mL water with a constant pressure dro...
Embodiment 2
[0060] The synthesis of the intermediate shown in embodiment 2 formula (II)
[0061]
[0062] In a 250mL three-necked bottle, add the intermediate shown in 50g of the formula (I) prepared in Example 1 and 50mL of methanol, stir, cool down to -5~5°C, and then add dropwise 69.4g of methanol-hydrogen chloride solution (the mass of hydrogen chloride Fraction is 25%), the temperature of the reaction system is controlled at 0~5°C during the dropwise addition, after the dropwise addition is completed, the temperature is raised to 25~30°C, after the reaction is stirred for 12 hours, a sample is taken for HPLC detection, mono-Boc(tert-butoxycarbonyl ) by-product is less than 1%, stop stirring, the reaction solution is added dropwise in 400mL ethyl acetate, a large amount of solids are separated out, filter, and wash the filter cake with ethyl acetate, dry, obtain solid 44.29g, and through routine The method for confirming the structure was confirmed to be an intermediate represented...
Embodiment 3
[0065] The synthesis of the intermediate shown in embodiment 3 formula (II)
[0066] In a 250mL three-necked bottle, add 50g of the intermediate of formula (I) prepared in Example 1 and 50mL of methanol, stir, and cool down to -5 to 5°C, then add dropwise 46.3g of methanol-hydrogen chloride solution (the mass of hydrogen chloride Fraction is 35%), during the dropwise addition process, the temperature of the reaction system is controlled at 0-5°C. After the dropwise addition, the temperature is raised to 25-30°C. After the reaction is stirred for 12 hours, a sample is taken for HPLC detection, and the single Boc by-product is less than 1%. , stop stirring, add the reaction solution dropwise to 400mL ethyl acetate, a large amount of solids precipitate out, filter, and wash the filter cake with ethyl acetate, dry to obtain 44.75g of solids, and through the conventional structure confirmation method, it is confirmed as The yield of the intermediate represented by the above formula...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com