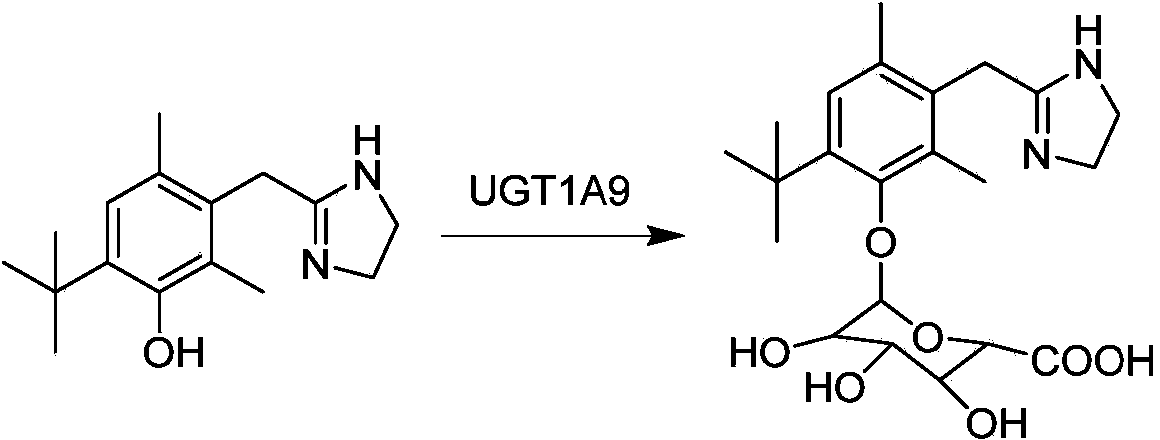
Application of oxymetazoline as special substrate for glucuronyl transferase UGT1A9
A technology of glucuronic acid and oxymetazoline, applied in the field of medicine, can solve the problem that the substrate does not have enzyme specificity, and achieve the effects of remarkable technical performance, good industrialization prospect and easy detection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
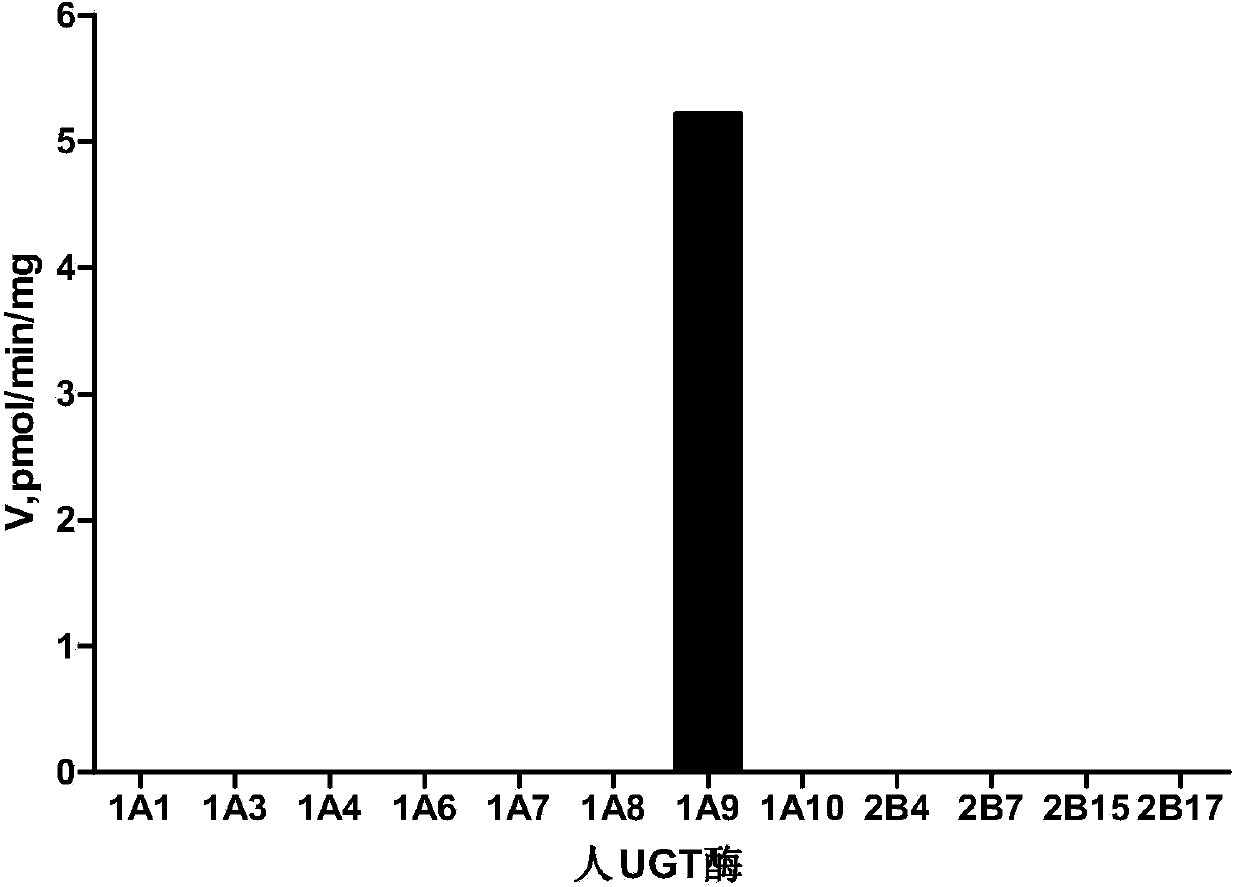
[0036] Embodiment 1 Oxymetazoline is used for measuring the enzymatic activity of single enzyme UGT1A9
[0037] (1) Prepare 300μL incubation system in advance, including 50mM phosphate buffer, 1mM MgCl 2 , 5mM Saccharolactone (glucaric acid monolactone), human UGT1A9 single enzyme (BD company, the United States) (final concentration is 0.05mg / mL), a series of concentrations of oxymetazoline (10mM, 5mM, 2.5mM, 1.25 mM, 0.625mM, 0.3125mM, 0.15625mM) and UDPGA with a final concentration of 2mM; pH 7.2-7.5. The preparation process of the system must be operated on ice, and finally UDPGA is added to the system to start the reaction;
[0038] 50mM phosphate buffer was prepared by KH 2 PO 4 and K 2 HPO 4 Prepared with a pH of 7.4;
[0039] (2) Transfer the incubation system to a constant temperature environment of 37°C for reaction. After 2 hours, add 100 μL of acetonitrile into the system and mix thoroughly to terminate the reaction;
[0040] (3) Under the centrifugation cond...
Embodiment 2
[0043] Example 2 Oxymetazoline Determination of UGT1A9 Enzyme Activity in Human Liver Microsomes
[0044] (1) The incubation system was as in Example 1, the enzyme in the system was replaced with human liver microsomes (BD Company, the United States), the final protein concentration of human liver microsomes in the system was 0.5 mg / mL, and the reaction time was 1 h;
[0045] (2) Sample processing and analysis As described in Example 1, the enzyme activity of UGT1A9 in liver microsomes was measured by the amount of glucuronidation product produced per unit time. The maximum catalytic rate of UGT1A9 enzyme in human liver microsomes was measured to be 7.53±0.52 pmol / min / mg.
[0046] The more metabolites generated per unit time, the stronger the UGT1A9 enzyme activity in human liver microsomes.
Embodiment 3
[0047] Example 3 Utilizing the correlation between the metabolic rate of oxymetazoline and propofol in individual human liver microsomes to evaluate the ability of UGT1A9 enzymes in human liver microsomes to dispose of glucuronidation of propofol
[0048] Using oxymetazoline and propofol (purchased from Sigma) as substrates, incubation reactions were carried out in 10 cases of individual human liver microsomes (BD Company, USA) with different UGT1A9 enzyme activities, incubation conditions and sample processing Analysis is the same as described in Example 1. The generation rate of the glucuronidation product of oxymetazoline / propofol in each case of human liver microsome reaction system was measured, and then statistical software (GraphPad Prism V5 software (SanDiego, CA)) was used to determine the rate of glucuronidation products of oxymetazoline and propofol, respectively. The generation rate of the glucuronidation product of pophenol is taken as the abscissa and ordinate, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com