Preparation method of alumina coated lithium cobaltate positive electrode material
A technology for coating lithium cobaltate and positive electrode materials, which is applied in the field of preparation of alumina-coated modified lithium cobaltate positive electrode materials, can solve the problems of cumbersome filtering and washing processes, reduced production efficiency, and increased costs, and achieve good battery life. Chemical cycle stability, damage reduction, uniform coating effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
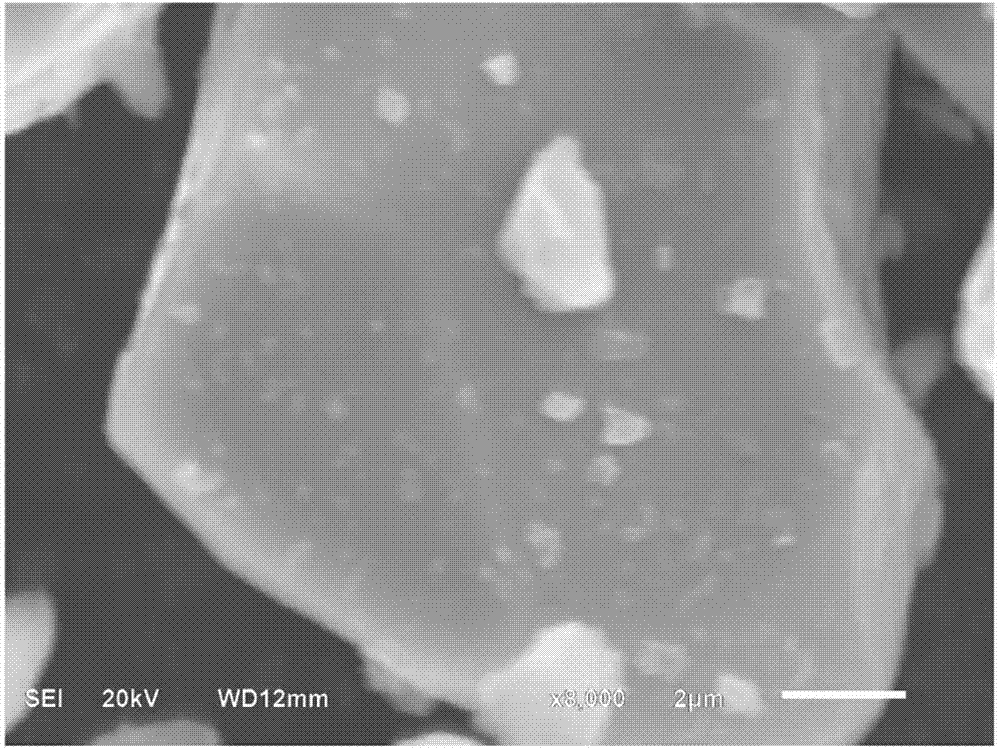
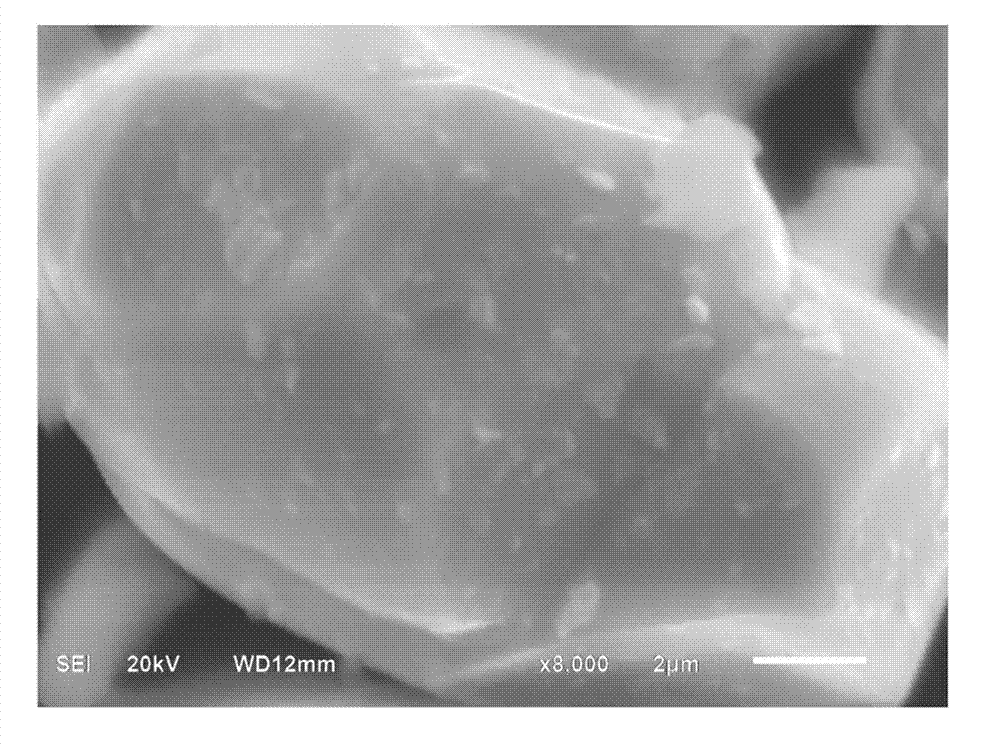
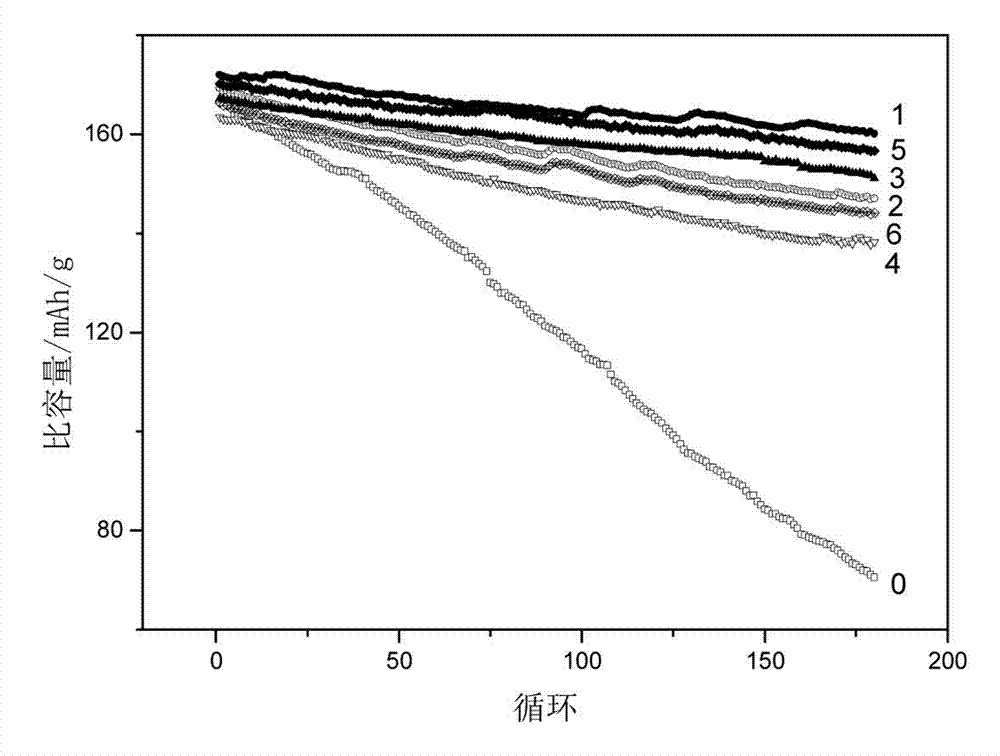
[0015] Calculated by mass ratio, namely Al 2 O 3 : LiCoO 2 =1:100 ratio, select aluminum isopropoxide as the aluminum source, calculate the amount of aluminum isopropoxide according to the required amount of aluminum oxide, and heat 100g lithium cobaltate and 4.0g aluminum isopropoxide in a converter to At 140°C, the aluminum isopropoxide was vaporized, kept for 2 hours, and then water vapor (approximately 500ml) was slowly introduced, and then rotated for 1 hour. The temperature was increased to 300°C and kept at a constant temperature for 3 hours, and then naturally cooled to room temperature to obtain the alumina of the invention. Coated LiCoO 2 The product, the alumina quality is LiCoO 2 1% of quality. From figure 2 It can be seen that the morphology of the lithium cobalt oxide particles modified by the method remains basically unchanged, that is, the morphology of the lithium cobalt oxide particles will not be damaged, and the excellent processing performance of the pure p...
Embodiment 2
[0017] Calculated by mass ratio, namely Al 2 O 3 : LiCoO 2 =1:200 ratio, select aluminum stearate as the aluminum source, calculate the amount of aluminum stearate according to the required amount of alumina, put 100g of lithium cobaltate and 8.60g of aluminum stearate in a converter and heat to 150°C, vaporize the aluminum stearate in it, keep it warm for 0.5h, then slowly pass in water vapor (about 500ml), then rotate for 1.5h, continue to heat up to 300°C and keep constant temperature for 4 hours, and then naturally cool to room temperature to obtain the present invention Alumina coated LiCoO 2 The product, the alumina quality is LiCoO 2 0.5% of quality.
Embodiment 3
[0019] Calculated by mass ratio, namely Al 2 O 3 : LiCoO 2 =1:125 ratio, select aluminum acetate as the aluminum source, calculate the amount of aluminum acetate according to the required amount of alumina, put 100g lithium cobaltate and 3.2g aluminum acetate in a converter and heat it to 220°C, so that The aluminum acetate is vaporized, kept for 1 hour, then water vapor (about 500ml) is slowly introduced, and then rotated for 2 hours, the temperature is continued to be increased to 400°C and kept at a constant temperature for 5 hours, and then naturally cooled to room temperature to obtain the aluminum oxide coated LiCoO of the present invention 2 The product, the alumina quality is LiCoO 2 0.8% of quality.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com