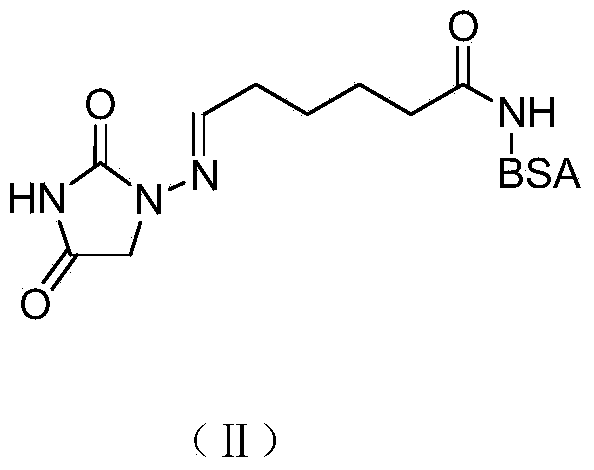
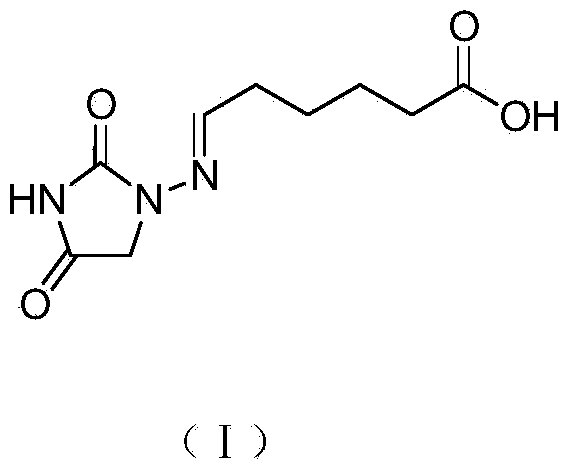
Preparation of immunizing antigen and envelope antigen for detecting nitrofurantoin metabolites
A technology for nitrofurantoin metabolites and immune antigens, applied in the field of detection of nitrofurantoin metabolites, can solve problems such as metabolite residues, and achieve the effects of improving immunogenicity, specificity, and coupling ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] 1 Synthesis of nitrofurantoin metabolite hapten by acetal method
[0021] 200 mL of ethanol was added into a 500 mL round bottom flask, nitrofurantoin (10.2 g, 0.1 mol) and 6-oxohexanoic acid (15.6 g, 0.12 mol) were added in sequence, and heated to reflux for 3 hours. Heating was stopped, and the reaction solution was naturally cooled to room temperature, and left to stand overnight. After suction filtration, the filter cake was washed with ethanol (20.0 mL×3), and dried at 50° C. for 5 hours to obtain 20.4 g of carboxyl-containing nitrofurantoin derivatives. Preparation of nitrofurantoin metabolite hapten (I).
[0022] 2 Synthesis of Nitrofurantoin Metabolite Immune Antigen by Active Thioester Method
[0023] Dissolve nitrofurantoin hapten (0.214g, 1.0mmol) in 30mL of dimethylformamide (Nimethylformamide, DMF), cool to 0-4°C, add 3-phosphorylated diphenyl ester benzo[D]oxane under stirring Azol-2-one [2(3H)-Benzoxazolone,3-(diphenoxyphosphinyl)-(9CI), DPBO] (0.351g,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com