Method for removing iron impurities and gathering cupric ions in copper sulfate solution
A technology of iron impurities and copper sulfate, applied in the direction of copper sulfate, etc., can solve the problems of affecting the working exchange capacity of ion exchange resin, resin poisoning failure, high process cost, etc., and achieve good iron removal effect, low production cost and simple process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
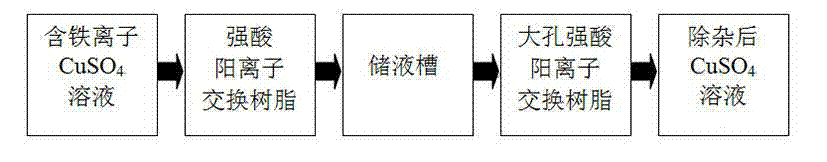
[0035] Example 1: A removal of CuSO 4 Iron impurities in solution and enriched Cu 2+ method, including the following steps:
[0036] CuSO with a concentration of 2.5g / L 4 Adjust the pH value of the crude solution to 2-4 with sulfuric acid, and use a corrosion-resistant infusion pump to dissolve CuSO 4 The crude solution is poured into a column equipped with 001×7 strong acid cation resin to remove Fe 3+ , Fe 2+ , when processing CuSO 4 The flow rate of the crude solution is 8BV / h, and the low-iron CuSO is obtained after treatment 4 solution;
[0037] Pass the low-iron copper sulfate solution into the macroporous strong acid cation exchange resin column at a flow rate of 2BV / h to absorb and enrich Cu 2+ , Cu in solution 2+ Ion exchange reaction with macroporous strong acid cation resin, Cu 2+ All are adsorbed by macroporous strong acid cation resin;
[0038] Adsorption of Cu by Macroporous Strong Acid Cationic Resin 2+ After saturation, use 18% sulfuric acid to bac...
Embodiment 2
[0040] Example 2: A removal of CuSO 4 Iron impurities in solution and enriched Cu 2+ method, including the following steps:
[0041] CuSO with a concentration of 3.0g / L 4 For the crude solution, adjust the pH value to 2-4 with sulfuric acid, and use a corrosion-resistant infusion pump to dissolve CuSO 4 The crude solution is poured into a column equipped with 001×7 strong acid cation resin to remove Fe 3+ , Fe 2+ , when processing CuSO 4 The flow rate of the crude solution was 10BV / h to obtain low-iron CuSO 4 solution;
[0042] Pass the low-iron copper sulfate solution into the macroporous strong acid cation exchange resin column at a flow rate of 4BV / h to absorb and enrich Cu 2+ , Cu in solution 2+ Ion exchange reaction with macroporous strong acid cation resin, Cu 2+ All are adsorbed by macroporous strong acid cation resin;
[0043] Adsorption of Cu by Macroporous Strong Acid Cationic Resin 2+ After saturation, use 18% sulfuric acid to backwash the resin at ...
Embodiment 3
[0045] Example 3: A removal of CuSO 4 Iron impurities in solution and enriched Cu 2+ method, including the following steps:
[0046] CuSO with a concentration of 4.5g / L 4 For the crude solution, use sulfuric acid to adjust the pH value to 2-4, and use a corrosion-resistant infusion pump to pour it into a column equipped with 001×7 strong acid cation resin to remove Fe. 3+ , Fe 2+ , when processing CuSO 4 The flow rate of the crude solution was 12BV / h to obtain low-iron CuSO 4 solution;
[0047] Pass the low-iron copper sulfate solution into the macroporous strong acid cation exchange resin column at a flow rate of 5BV / h to adsorb and enrich Cu 2+ , Cu in solution 2+ Ion exchange reaction with macroporous strong acid cation resin, Cu 2+ All are adsorbed by macroporous strong acid cation resin;
[0048] Adsorption of Cu by Macroporous Strong Acid Cationic Resin 2+ After saturation, backwash with 18% sulfuric acid at a flow rate of 3BV / h for desorption, and obtain a d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com