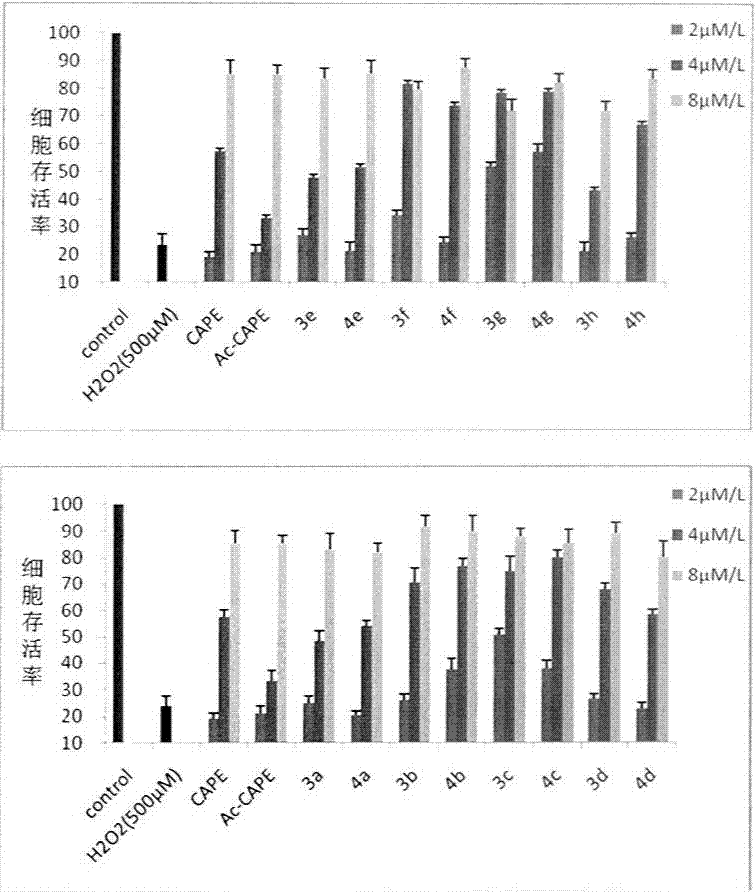
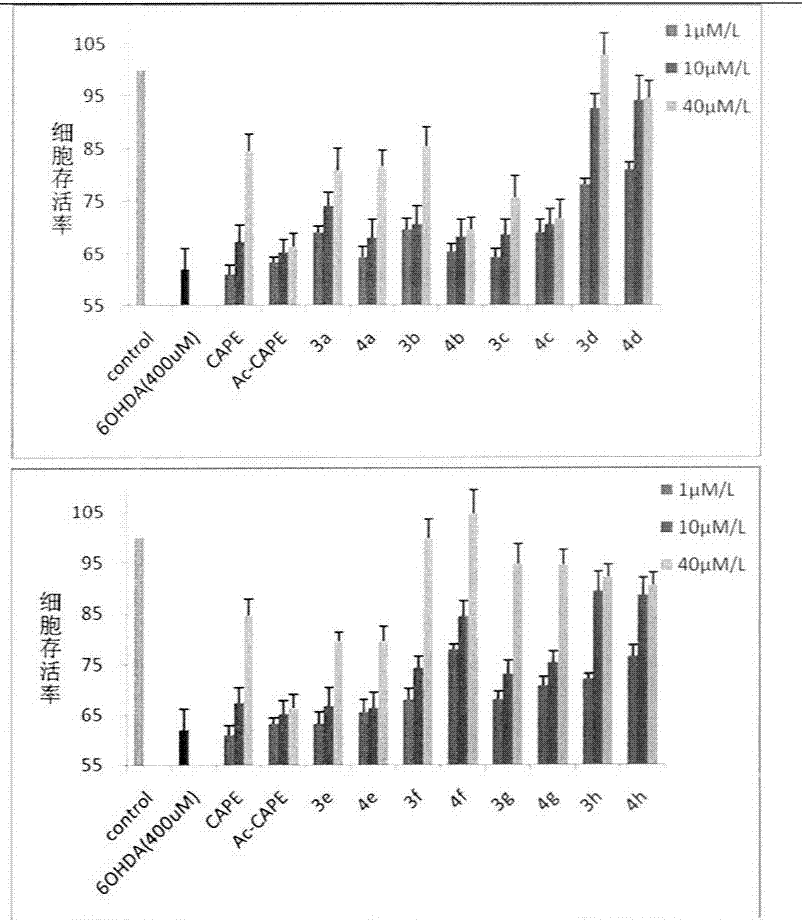
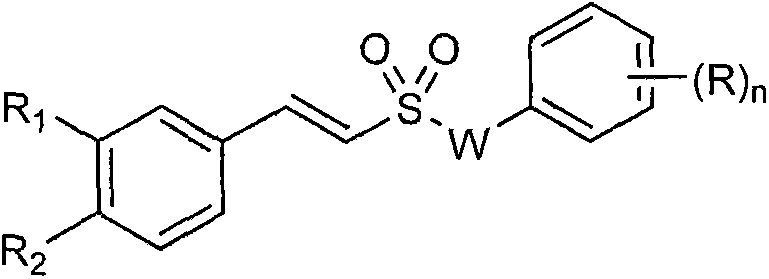
Styryl sulfone compounds, their preparation and their use as neuroprotective agents
A compound and alkyl technology, applied in the field of E-3, can solve the problems of not easily permeating the blood-brain barrier and fast metabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1: Preparation of E-2-(3,4-dihydroxy)styrylbenzyl sulfone (3a)
[0059] Step 1, the preparation of 2-benzylthioacetic acid (1a)
[0060]
[0061] Thioglycolic acid (0.69ml, 10mmol) was dissolved in a reaction flask filled with 15ml of methanol, NaOH (0.8g, 20mmol) in methanol (10ml) was added dropwise, followed by benzyl bromide (1.19ml, 10mmol) dropwise, Stir at room temperature for 6 hours, TLC detected that the reaction was complete, spin the solvent under reduced pressure, add a small amount of water, neutralize with 1N hydrochloric acid to pH 7, extract with ethyl acetate, wash the combined ester layer with saturated brine, and dry over anhydrous sodium sulfate. Separation and purification by silica gel column (petroleum ether / ethyl acetate elution) gave white solid 1a, 1.7 g, yield 93.4%, m.p.62-63°C.
[0062] 1 H NMR (400MHz, DMSO-d 6 )δ=12.61(s, 1H, COOH), 7.24-7.35(m, 5H, ArH), 3.81(s, 2H, SCH 2 COOH), 3.12(s, 2H, ArCH 2 S).
[0063] Step 2,...
Embodiment 2
[0073] Example 2: Preparation of E-2-(3,4-diacetoxy) styrylbenzyl sulfone (4a)
[0074]
[0075] Compound E-2-(3,4-dihydroxy)styrylbenzyl sulfone (0.19g, 0.66mmol) was dissolved in acetic anhydride (10ml), anhydrous pyridine (0.11ml, 1.34mmol) was added, and the reaction was stirred After 5 minutes, TLC detected that the reaction was complete, poured the reaction solution into ice water and stirred, extracted with ethyl acetate, washed the ester layer with saturated brine, dried over anhydrous sodium sulfate, separated and purified with silica gel column (petroleum ether / ethyl acetate elution) A white cotton-like solid 4a was obtained, 220mg, 89.8% yield, m.p.163-164°C.
[0076] 1 H NMR (400MHz, CDCl 3 ) δ=7.24-7.41 (m, 9H, ArH, ArCH=CHSO 2 ), 6.66 (d, J=15.2Hz, 1H, ArCH=CHSO 2 ), 4.32 (s, 2H, ArCH 2 SO 2 ), 2.33 (s, 3H, p-ArOCOCH 3 ), 2.32 (s, 3H, m-ArOCOCH 3 ).
[0077] 13 C NMR (100MHz, CDCl 3 )δ=167.98, 167.88, 144.36, 143.66, 142.52, 130.93, 129.04, 128.97, ...
Embodiment 3
[0079] Example 3: Preparation of E-2-(3,4-dihydroxy)styryl-4-chlorobenzyl sulfone (3b)
[0080] Step 1, the preparation of 2-(4-chloro)benzylthioacetic acid (1b)
[0081]
[0082] Using the synthesis method of compound 1a above, 4-chlorobenzyl bromide was used as a reactant to obtain white solid 1d with a yield of 85.5%, m.p.51-52°C.
[0083] Step 2, the preparation of 2-(4-chloro)benzylsulfonylacetic acid (2b)
[0084]
[0085] Using the synthesis method of compound 2a above, 2-(4-chloro)benzylthioacetic acid was used as a reactant to obtain white solid 2d with a yield of 82.1%, m.p.144-145°C.
[0086] Step 3: Preparation of E-2-(3,4-dihydroxy)styryl-4-chlorobenzyl sulfone (3b)
[0087]
[0088] Using the synthesis method of compound 3a above, 2-(4-chloro)benzylsulfonylacetic acid was used as a reactant to obtain white solid 3b with a yield of 71.1%, m.p.166-167°C.
[0089] 1 H NMR (400MHz, DMSO-d 6 )δ=9.75(s, 1H, p-ArOH), 9.23(s, 1H, m-ArOH), 6.76-7.45(m, 9H, A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com