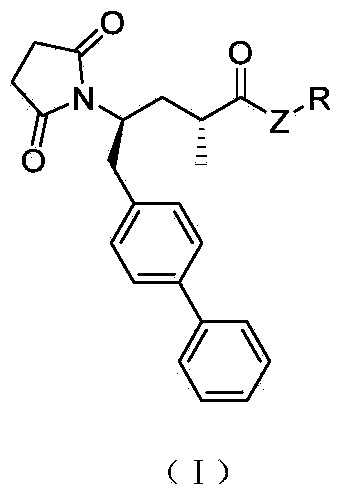
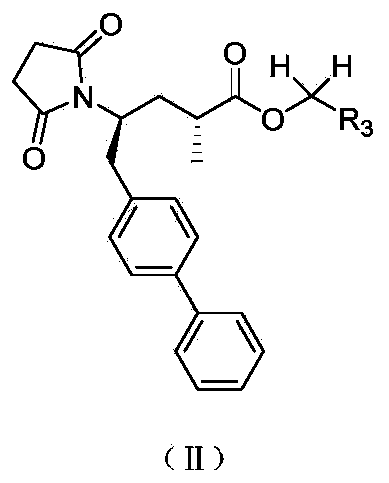
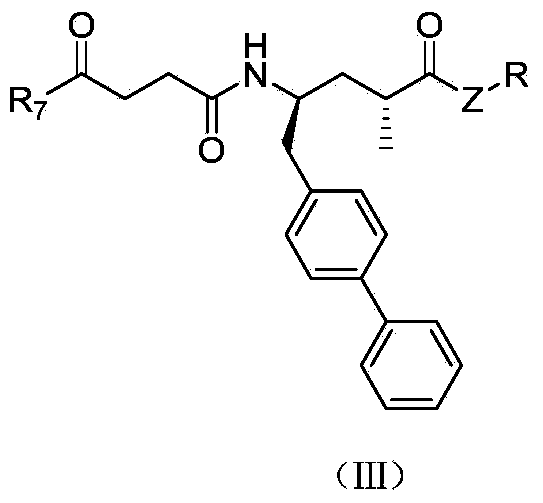
Biaryl-substituted 4-aminobutyric acid derivatives, and preparation method and application thereof
A technology of aminobutyric acid and biaryl, applied in the field of biaryl-substituted 4-aminobutyric acid derivatives and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Preparation of (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methylpentanoic acid ethoxycarboxymethyl ester
[0115]
[0116] Add L-pyroglutaminol (50.0g, 0.43mol) into 500mL of dichloromethane, and after cooling in an ice-water bath, add triethylamine (120mL, 0.86mol) and p-toluenesulfonyl chloride (90.0g, 0.47mol) successively, at room temperature React overnight. After filtration and concentration, the crude product was recrystallized from petroleum ether-ethyl acetate to obtain (S)-5-carbonyl-pyrrolidin-2-ylmethyltoluene-4-sulfonate (110.0 g, 95%) as an off-white solid.
[0117]
[0118] Weigh magnesium chips (10.9g, 0.45mol) and place them in a 2000mL three-necked flask, and dissolve 4-bromobiphenyl (93.2g, 0.40mol) in 400mL tetrahydrofuran, then slowly drop them into the three-necked flask for about two hours. After the addition, the reflux reaction was continued for two hours. After cooling to room temperature, 25 mL of 1,4-dioxane was added, the...
Embodiment 2
[0170] Preparation of (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methylpentanoic acid cyclohexyloxycarboxymethyl ester
[0171]
[0172] Cyclohexanol (2.50g, 25.0mmol) was added to 25mL of dichloromethane, and cooled in an ice-water bath; chloromethyl chloroformate (2.2mL, 25.0mmol) was dissolved in 25mL of dichloromethane, and slowly added dropwise to the reaction system; pyridine (2.2mL , 27.5mmol) was diluted in 5mL of dichloromethane, and then slowly added dropwise to the reaction system. React overnight at room temperature. Add dichloromethane for dilution, wash with water three times, and dry over anhydrous sodium sulfate. Concentration gave cyclohexyl chloromethyl carbonate (4.80 g, 99%) as a colorless liquid.
[0173] 1 H NMR (400MHz, CDCl 3 )δ5.66(s,2H),4.72-4.58(m,1H),1.93-1.80(m,2H),1.76-1.63(m,2H),1.54-1.39(m,3H),1.37-1.15( m,3H).
[0174]
[0175] Cyclohexyl chloromethyl carbonate (4.8g, 25.0mmol) was dissolved in 25mL of acetonitrile, then...
Embodiment 3
[0185] Preparation of (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methylpentanoic acid isopropoxycarboxymethyl ester
[0186]
[0187] Isopropanol (1.9mL, 25.0mmol) was added to 25mL of dichloromethane and cooled in an ice-water bath; chloromethyl chloroformate (2.2mL, 25.0mmol) was dissolved in 25mL of dichloromethane and slowly added dropwise to the reaction system; pyridine (2.2mL , 27.5mmol) was diluted in 5mL of dichloromethane, and then slowly added dropwise to the reaction system. React overnight at room temperature. Add dichloromethane for dilution, wash with water three times, and dry over anhydrous sodium sulfate. Concentration gave chloromethyl isopropyl carbonate (3.8 g, 99%) as a colorless liquid.
[0188] 1H NMR (400MHz, CDCl 3 )δ5.66(s,2H),4.89(m,1H),1.27(d,6H).
[0189]
[0190] Isopropyl chloromethyl carbonate (3.8g, 25.0mmol) was dissolved in 25mL of acetonitrile, and then sodium iodide (18.7g, 125.0mmol) was added, and reacted at room t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com