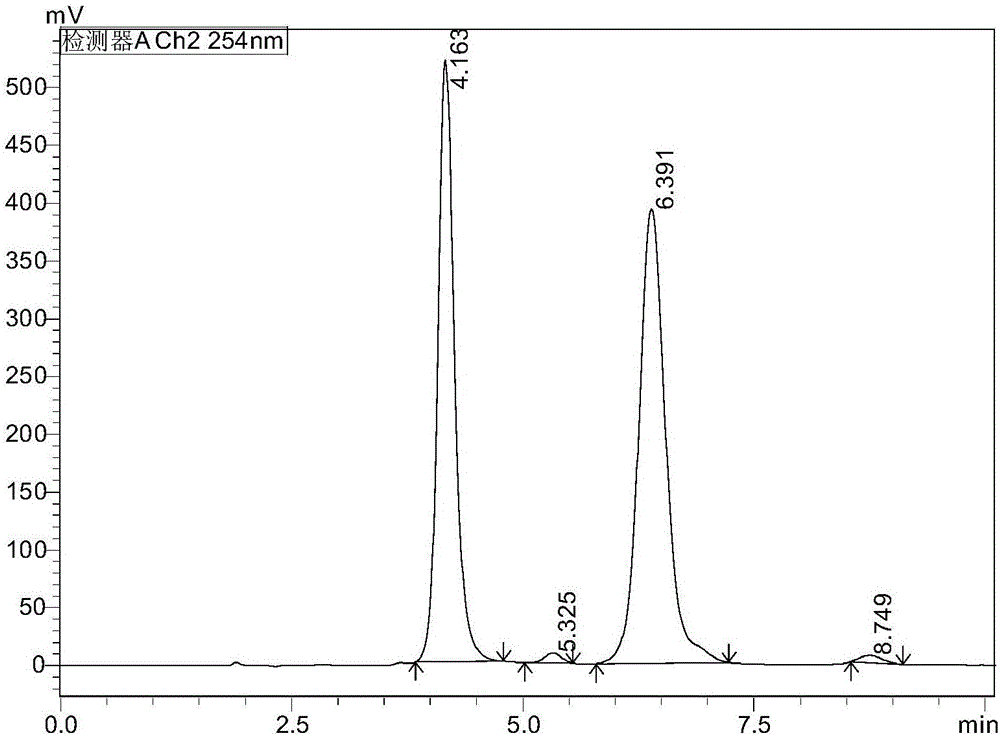
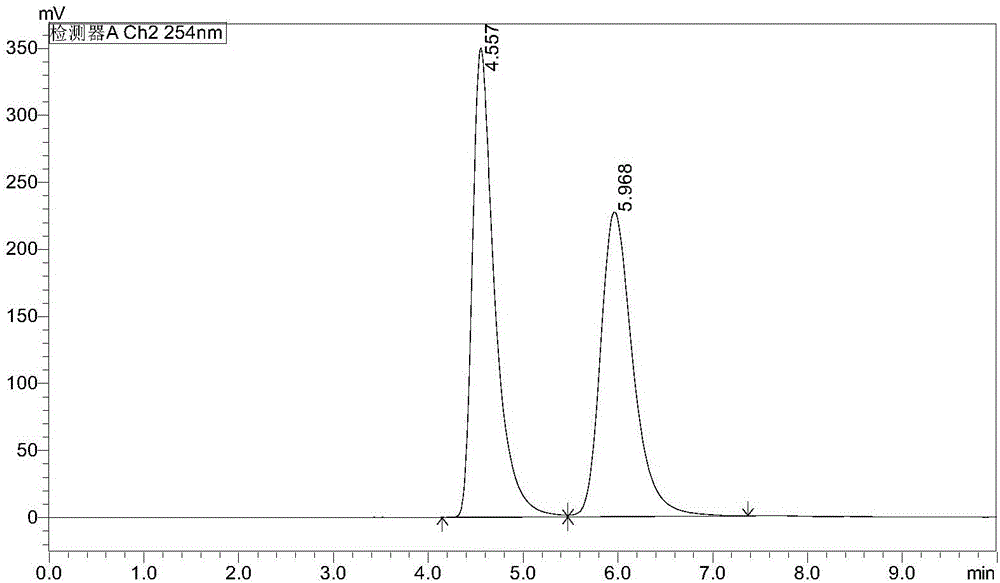
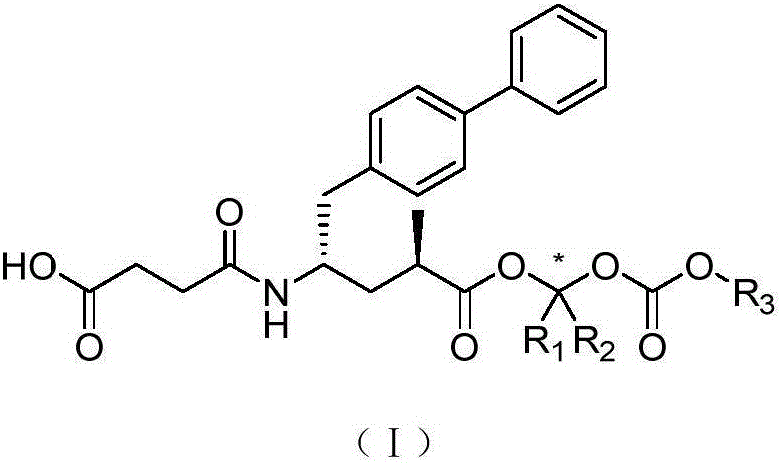
NEP inhibitor and medicine composition thereof
A technology for solvates and compounds, which is applied in the directions of drug combination, separation of optically active compounds, and pharmaceutical formulations to achieve the effects of high bioavailability, good solubility, and good pharmacokinetic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] The first step: the preparation of 1-chloroethyl isopropyl carbonate
[0070]
[0071] Compound 1-chloroethyl chloroformate (3ml, 27.8mmol) was diluted with DCM (25ml), cooled to 0°C, and isopropanol (2.1ml, 27.8mmol) diluted with DCM (25ml) was dropped with constant pressure Slowly add the liquid funnel, and react for about 15 minutes. Pyridine (2.4ml, 30.6mmol) diluted with DCM (5ml) is also slowly added using the constant pressure dropping funnel. Overnight, then diluted with water, extracted with DCM, the organic phase was dried and concentrated to obtain 3.74 g of light yellow liquid with a yield of 81%.
[0072] 1 HNMR: (400MHz, CDCl 3 ), 6.36(q,1H), 4.88(m,1H), 1.759(dm,3H), 1.26(t,6H).
[0073] The second step: the preparation of 1-iodoethyl isopropyl carbonate
[0074]
[0075] Compound 1-chloro-ethyl ester isopropyl carbonate (3.74g, 22.4mmol) was dissolved in acetonitrile (20ml), sodium iodide (16.8g, 112.2mmol) was added, and the temperature was ra...
Embodiment 2
[0100] The first step: (9R,11S)-11-([1,1'-biphenyl]-4-ylmethyl)-6-isopropyl-2,9-dimethyl-4,8,13 - Preparation of tricarbonyl-3,5,7-trioxa-12-azahexadecane-16-acid
[0101]
[0102] Compound (2R,4S)-4-(3-benzyloxycarbonyl-propionylamino)-5-biphenyl-4-yl-2-methylpentanoic acid (1410mg, 2.98mmol) was dissolved in 10mL N,N -Dimethylformamide with addition of Cs 2 CO 3 (580mg, 1.79mmol), stirred at room temperature for 1 hour, added KI (1240mg, 7.44mmol), 1-chloro-2-methylpropyl isopropyl carbonate (1450mg, 7.44mmol), heated at 65°C for 24 hours. Reversed-phase column chromatography separated 745 mg of crude product (containing ring-closing by-products). It was then dissolved in 10 mL of methanol, 80 mg of palladium / carbon was added, and hydrogenated at room temperature and pressure for 5 hours. Filtration, concentration, separation by reverse phase column chromatography, white foamy solid (9R,11S)-11-([1,1'-biphenyl]-4-ylmethyl)-6-isopropyl-2 , 9-Dimethyl-4,8,13-tricarbony...
experiment example 1
[0116] (Rat pharmacokinetic study test)
[0117] 1. Research purpose
[0118] Taking rats as test animals, study the pharmacokinetic behavior of NEP series prodrug compound (6), compound (1-6R), compound (2-6R), compound (2-6S) in rats, and evaluate its pharmacokinetic characteristics.
[0119] 2. Test plan
[0120] 2.1 Test animals
[0121] Three SD rats, male, were used in each case, provided by Xipuer-Bikai Experimental Animal Co., Ltd., animal production license number SCXK (Shanghai) 2008-0016.
[0122] 2.2 Drug preparation
[0123] Take all the samples (according to 30mg), add 1ml of ethanol to dissolve, then add 1ml of Solutol HS15 and 8ml of pH 4.63 acetate buffer in order to make a 3mg / ml solution, and keep the remaining liquid as a sample for quantitative analysis.
[0124] 2.3 Administration
[0125] Three SD rats, male, were administered intragastrically after fasting overnight, with a dose of 30 mg / kg and a volume of 10 ml / kg.
[0126] 2.4 Sample Collection ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com