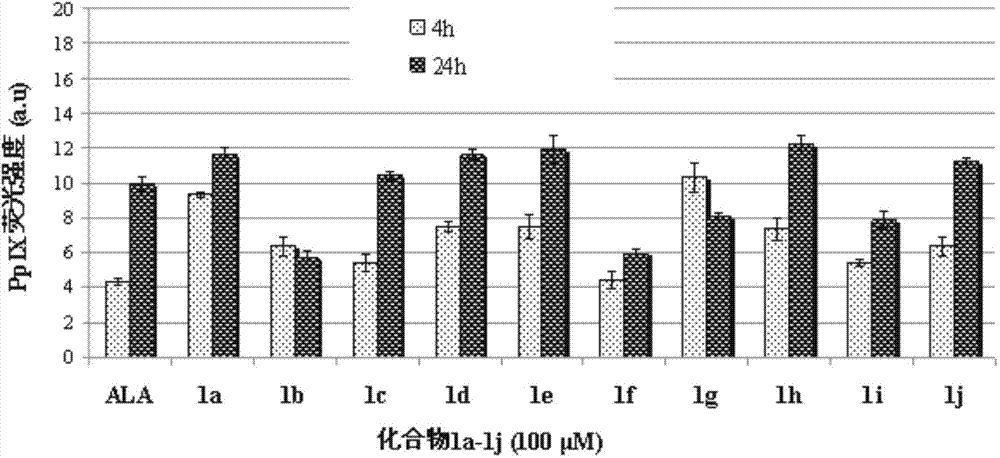
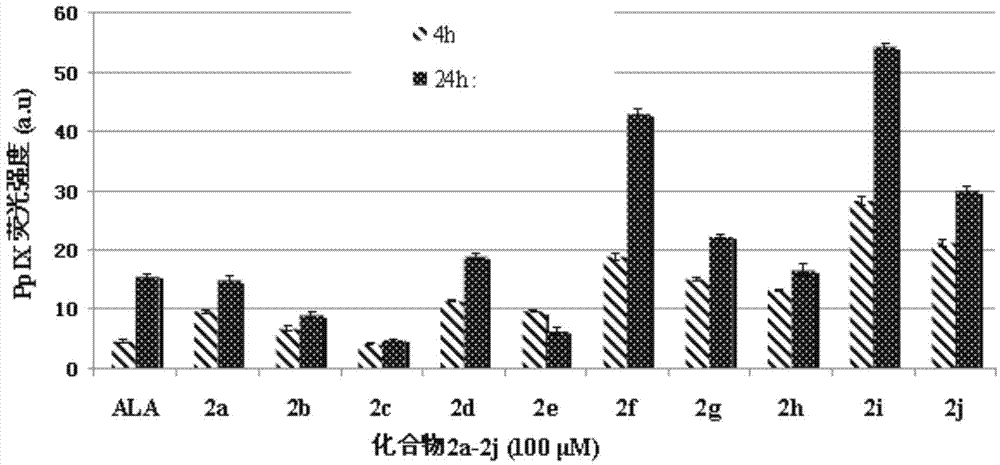
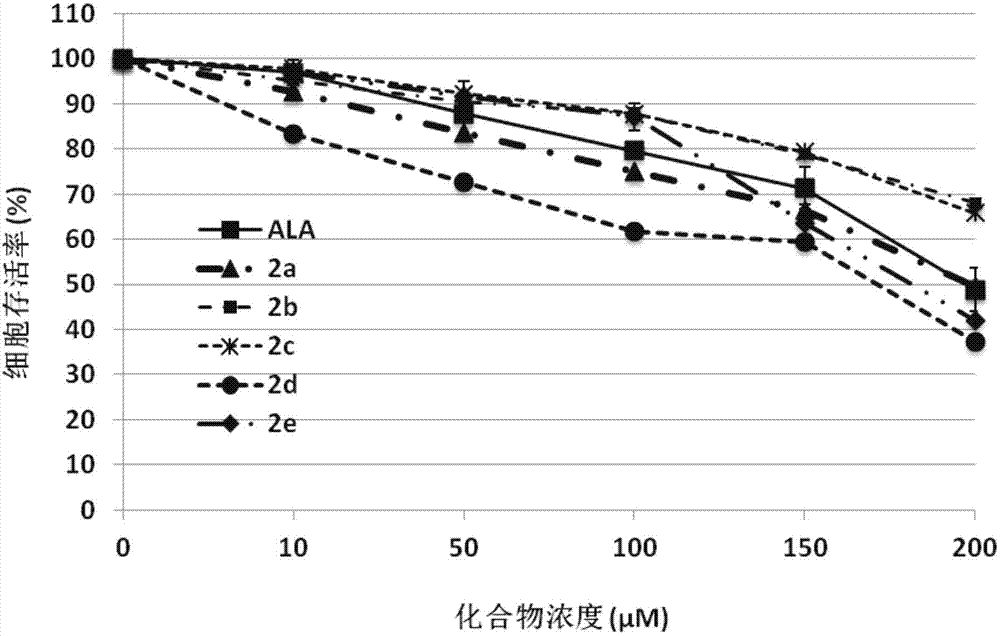
Conjugates of 5-aminolevulinic acid and 3-hydroxypyridin-4-one, preparing method thereof and uses of the conjugates
A technology of aminolevulinic acid and hydroxypyridine, which is applied in the field of photodynamic therapy drugs, 5-aminolevulinic acid-iron chelator conjugates and its preparation, and can solve the problems of increasing the concentration of PpIX
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1, the preparation method of compound 1, carry out the following steps successively:
[0052] A, the synthesis of compound 6 containing free carboxyl,
[0053] One, the synthesis of compound 6a, carry out the following steps successively:
[0054] ①. Weigh 0.2g (1.1mmol) methyl 5-aminolevulinate hydrochloride (3), 0.305g (1mmol) N-benzyloxycarbonyl-L-phenylalanine (4a) and 6-chlorobenzene Triazole-1,1,3,3-tetramethyluronium hexafluorophosphate (HCTU, 0.414g, 1mmol) was dissolved in N,N-dimethylformamide (DMF, 4mL), and then 0.301 g (3mmol) triethylamine, stirred overnight at room temperature (12h), evaporated the solvent (DMF), dissolved the residue with dichloromethane (30mL), washed with 5% (mass%) sodium bicarbonate aqueous solution, anhydrous sodium sulfate After drying, the solvent (dichloromethane) was distilled off, and the residue was purified by silica gel column chromatography (eluted first with cyclohexane: ethyl acetate = 1:1 volume ratio, after...
Embodiment 2
[0085] Embodiment 2, the preparation of compound 2
[0086] Dissolve 100mg of compound 1 in 2mL of pyridine, cool to ≤5°C in an ice bath, add 0.4mL of acetic anhydride, stir in an ice bath for 15 minutes, add 2mL of ice water to the reaction solution, freeze-dry (i.e., in a vacuum freeze dryer Drying in medium temperature for 24h), compound 2 was obtained quantitatively.
[0087] instruction manual:
[0088] When it is compound 1a, it corresponds to compound 2a;
[0089] When it is compound 1f, the corresponding compound 2f is obtained;
[0090] When it is compound 1i, the corresponding compound 2i is obtained;
[0091] When it is compound 1j, the corresponding compound 2j is obtained;
[0092] And so on for the rest.
[0093] Compound 2a: 1 H NMR (CD 3 OD, 500MHz) δ1.52 (t, J=7.5Hz, 3H, CH 3 ),2.01(s,3H,CH 3 ), 2.63(t, J=6.5Hz, 2H, CH 2 ),2.75(t,J=6.5Hz,2H,CH 2 ),2.91and3.19(m,2H,CH 2 ),4.04(m,2H,CH 2 ), 4.38 (q, J=7.5Hz, 2H, CH 2 ),4.67(m,1H,CH),5.38(s,2H,CH 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com