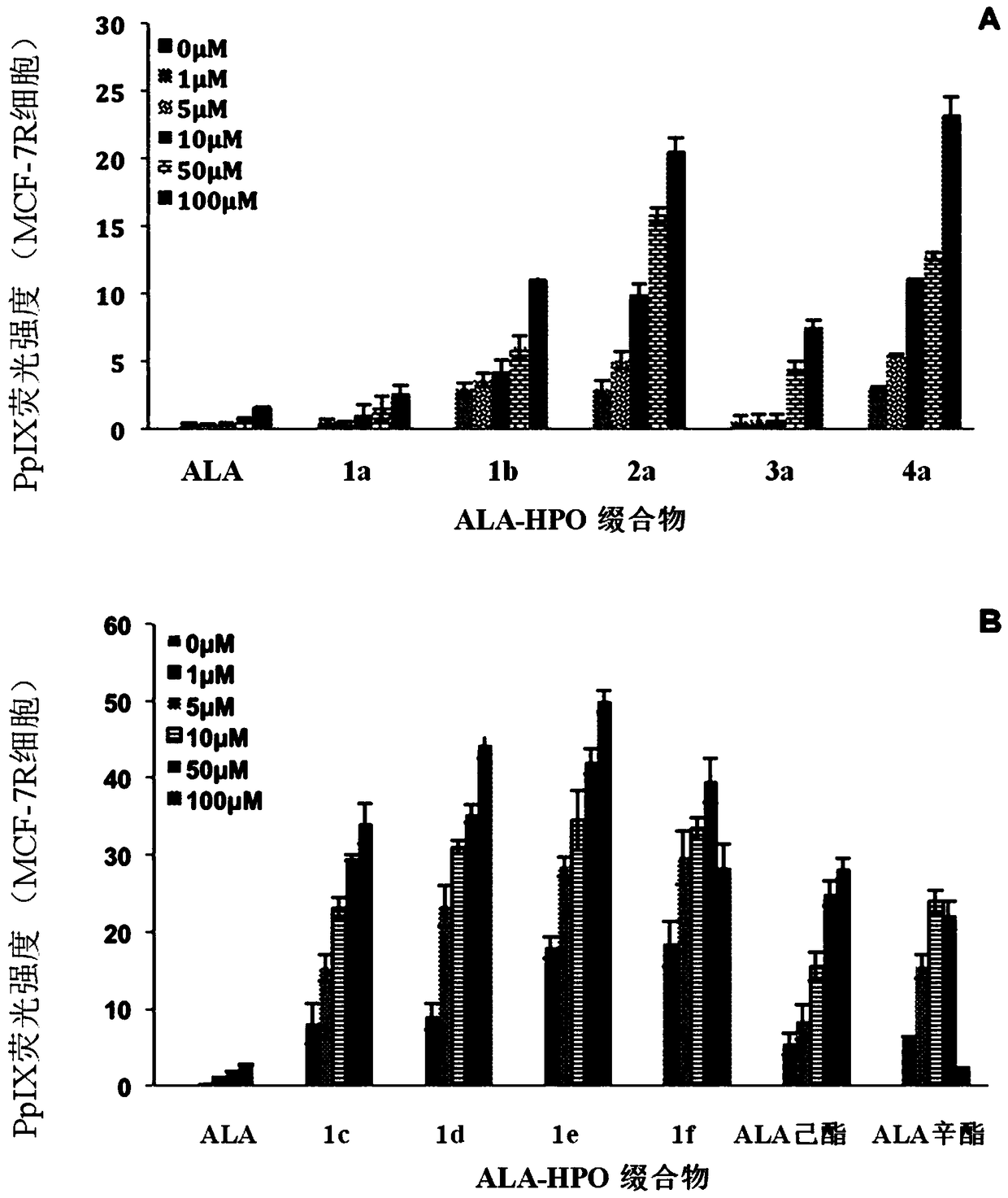
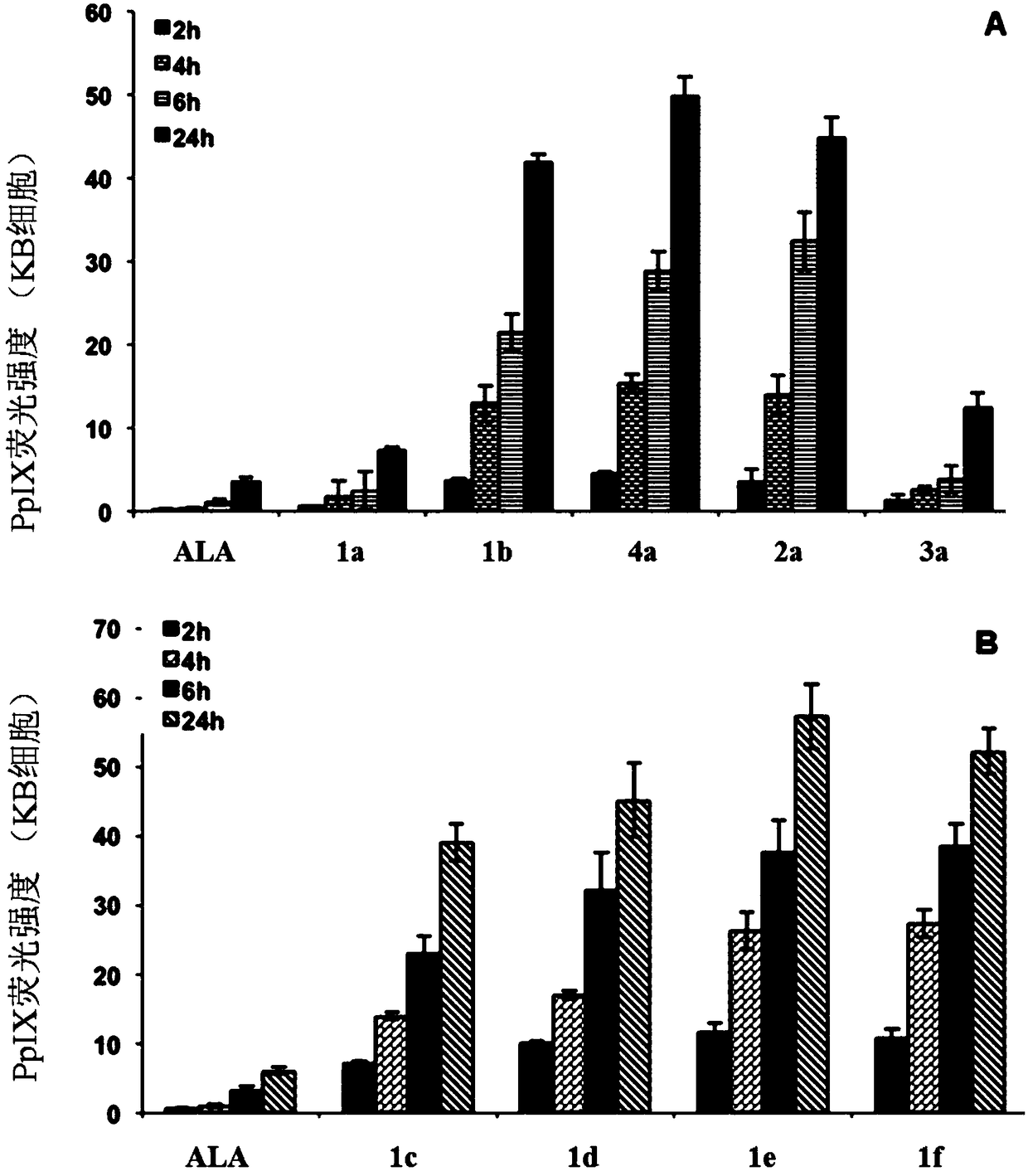
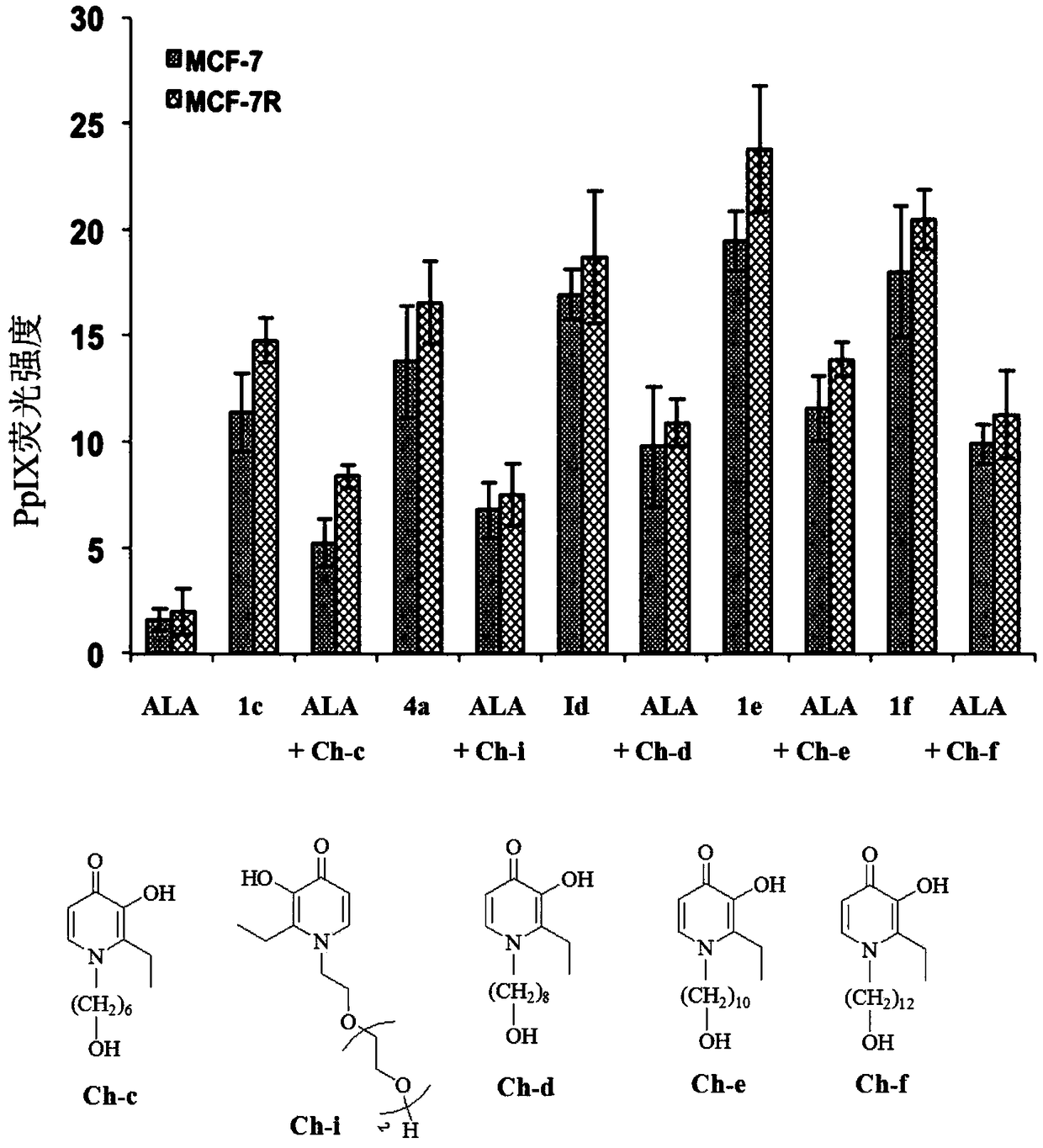
5-aminolevulinic acid/3-hydroxypyridone conjugate and its preparation method and use
A technology of aminolevulinic acid and hydroxypyridine, which can be used in pharmaceutical combinations, medical preparations containing active ingredients, and production of bulk chemicals, etc. Strong phototoxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1, the preparation method of compound 1, carry out the following steps successively:
[0046] A, the synthesis of compound 6
[0047] Benzylation of ethyl maltol: ethyl maltol - compound 5 (84g, 0.4mol) was dissolved in methanol (300mL), NaOH solution (26.4g dissolved in 80mL H 2 O). The mixture was heated to boiling, and benzyl bromide (85.6 mL, 0.72 mol) was added dropwise over 2 hours. Dissolved and continued heating at 60-65°C overnight (12h). NaBr was removed by filtration, the filtrate was concentrated to about 1 / 3 of the original volume with a rotary evaporator), dichloromethane (400 mL) was added, and 5% (mass %) NaOH solution (3 × 250 mL), H 2 O (2×300mL) washed with anhydrous Na 2 SO 4 dry. After filtration, the obtained filtrate was evaporated to remove the solvent (mainly dichloromethane, including methanol), added diethyl ether (100 mL), and placed in a 4°C refrigerator. The product (compound 6, ie, benzyl-protected ethyl maltol) was preci...
Embodiment 2
[0077] Embodiment 2, the preparation method of compound 2a, carry out the following steps successively:
[0078] A. Synthesis of Compound 10:
[0079] Benzyl-protected ethyl maltol—a mixture of compound 9 (10g, 43.4mmol), selenium dioxide (14.5g, 130.4mmol) and bromobenzene (50mL) was vigorously stirred at 135-140°C for 4h. After cooling and filtering, the filtrate was evaporated to dryness, and the residue was chromatographically purified on a silica gel column (with about 600 g of 200-300 mesh silica gel inside) (ethyl acetate / cyclohexane, 2:1) to obtain a brown oil --- Compound 10 ( 4.6g, 43%). 1 H NMR (CDCl 3 ,400MHz): δ1.23(d,J=6.7Hz,3H,CH 3 ), 4.85(q, J=6.7Hz, 1H, CH), 5.22(d, J=2.0Hz, 2H, CH 2 ), 6.46(d, J=5.6Hz, 1H, C5-H in pyridone), 7.37(m, 5H, Ph), 7.71(d, J=5.6Hz, 1H, C6-H in pyridone).ESI -MS:m / z 247([M+H] + ).
[0080] B. Synthesis of Compound 11:
[0081] Compound 10 (4.0g, 16.2mmol) was dissolved in dichloromethane (50mL), and 50% KOH (5.45g, 48.6mmol) ...
Embodiment 3
[0091] Embodiment 3, the preparation method of compound 3a, carry out the following steps successively:
[0092] A. Synthesis of Compound 17:
[0093] Compound 16 (0.94g, 3.82mmol) and N-hydroxysuccinimide (0.485g, 4.2mmol) were dissolved in tetrahydrofuran (20ml), stirred at room temperature for 30min, DCC (0.866g, 4.2mmol) was added, and stirred for 5h. A solution of methylamine in tetrahydrofuran (2M, 4.58mmol) was added and stirred overnight at room temperature. Filtration, the filtrate was concentrated, and purified by silica gel column chromatography (containing about 150 g of 200-300 mesh silica gel) (ethyl acetate / methanol 9:1) to obtain product 17 (0.85 g, 86%). 1 H NMR (CDCl 3 ,400MHz)δ2.78(d,J=5.0Hz,3H,CH 3 ),5.39(s,2H,CH 2 ), 6.49(d, J=5.6Hz, 1H, C5-H in pyridone), 7.40(m, 5H, Ph), 7.70(br, 1H, NH), 7.84(d, J=5.6Hz, 1H, C6-H in pyridone).ESI-MS: m / z260([M+H] + ).
[0094] Note: Compound 16 was synthesized according to literature method (Xie, Y.Y.; Liu, M.S.;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com