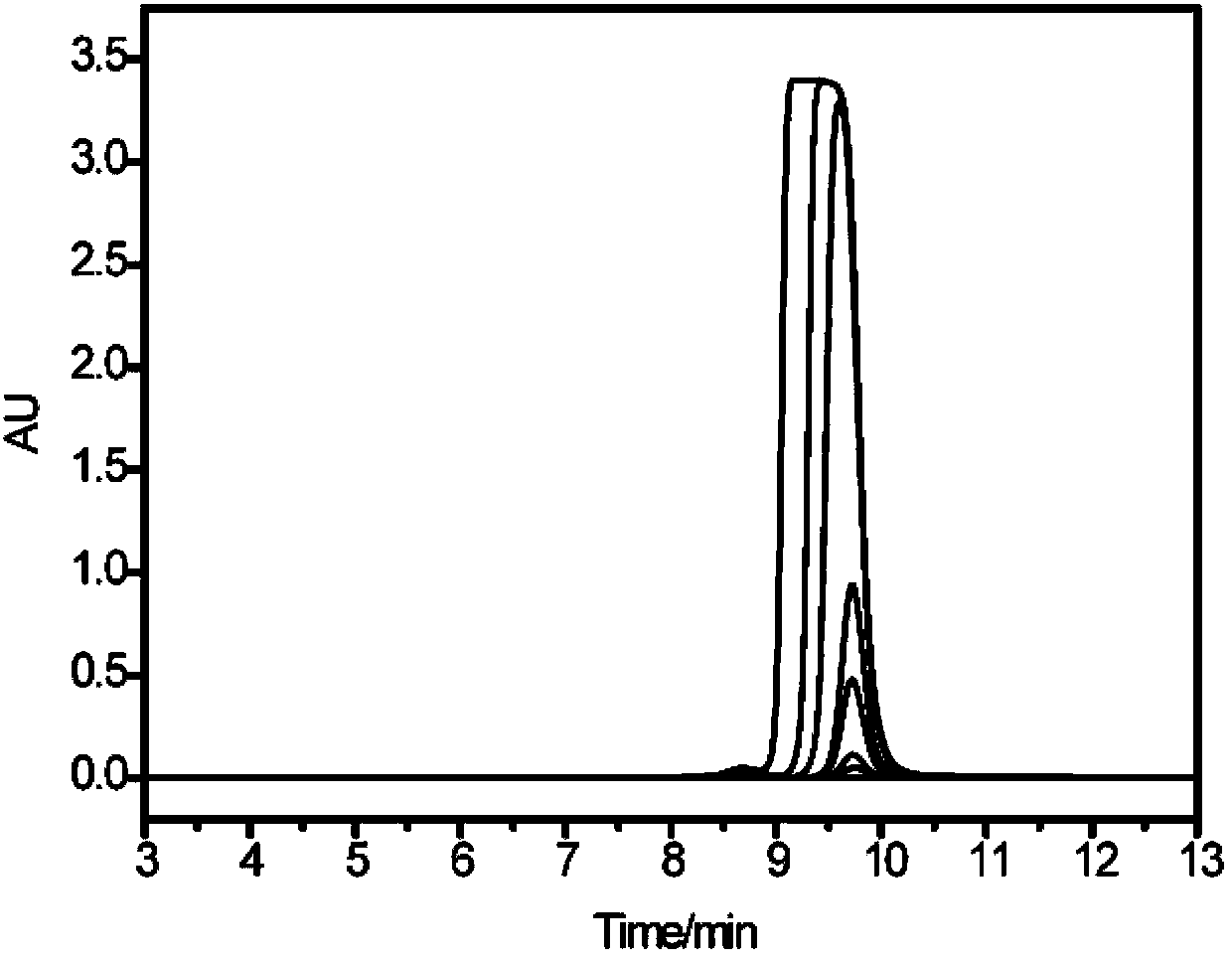
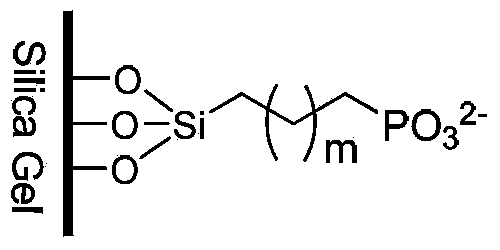
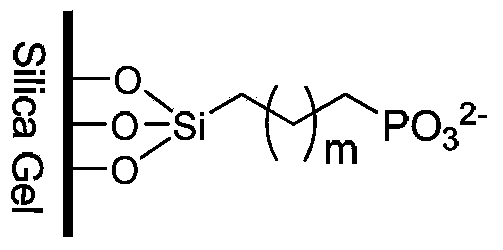
Cation exchange chromatography stationary phase and preparation method thereof
A chromatographic stationary phase and cation exchange technology, applied in the field of new liquid chromatography stationary phase and its preparation, can solve the problem of less silica matrix, and achieve the effects of good separation selectivity, novel structure and industrialization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Weigh 10g spherical silica gel (particle size is 5μm, pore size is 10nm, specific surface area is 305m 2 / g), placed in a 250mL glass flask, added 150mL of 10% hydrochloric acid solution, heated to reflux for 12 hours, cooled to room temperature, filtered, washed with water until neutral, and dried at 150°C for 24 hours. Under the condition of blowing dry nitrogen, 80 mL of dry toluene was added to the hydrated silica gel, stirred evenly, and then 6 mL of diethylphosphorylethyltriethoxysilane was added dropwise, and the reaction was stirred at 50°C for 24 hours. The reaction system was filtered, washed successively with toluene, dichloromethane, methanol, water, tetrahydrofuran, and methanol, and the product was dried at 60°C for 12 hours. Take 10g of the above-mentioned bonded silica gel, put it in a 250mL glass flask, add 100ml of 10% hydrochloric acid aqueous solution, stir at 50°C for 12 hours, cool the reaction system to room temperature, filter under reduced press...
Embodiment 2
[0027] Weigh 10g spherical silica gel (particle size is 5μm, pore size is 10nm, specific surface area is 305m 2 / g), placed in a 250mL glass flask, added 150mL of sulfuric acid solution with a volume concentration of 10%, heated to reflux for 12 hours, cooled to room temperature, filtered, washed with water until neutral, and dried at 150°C for 24 hours. Under the condition of blowing dry nitrogen, 80 mL of dry toluene was added to the silica gel, stirred evenly, then 6 mL of diethylphosphorylethyltriethoxysilane was added dropwise, and the reaction was stirred at 50°C for 24 hours. The reaction system was filtered, washed successively with toluene, dichloromethane, methanol, water, tetrahydrofuran, and methanol, and the product was dried at 60°C for 12 hours. Take 10g of the above-mentioned bonded silica gel, put it in a 250mL glass flask, add 100ml of 10% sulfuric acid aqueous solution, stir at 50°C for 12 hours, cool the reaction system to room temperature, filter under red...
Embodiment 3
[0029] Weigh 10g spherical silica gel (particle size is 5μm, pore size is 10nm, specific surface area is 305m 2 / g), placed in a 250mL glass flask, added 150mL of 10% hydrochloric acid solution, heated to reflux for 12 hours, cooled to room temperature, filtered, washed with water until neutral, and dried at 150°C for 24 hours. Under the condition of blowing dry nitrogen, 80 mL of dry toluene was added to the silica gel, stirred evenly, and then 6 mL of diethylphosphorylethyltriethoxysilane was added dropwise, and the reaction was stirred at 70°C for 24 hours. The reaction system was filtered, washed successively with toluene, dichloromethane, methanol, water, tetrahydrofuran, and methanol, and the product was dried at 60°C for 12 hours. Take 10g of the above-mentioned bonded silica gel, put it in a 250mL glass flask, add 100ml of 70% hydrochloric acid aqueous solution, stir at 50°C for 12 hours, cool the reaction system to room temperature, filter under reduced pressure and w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com