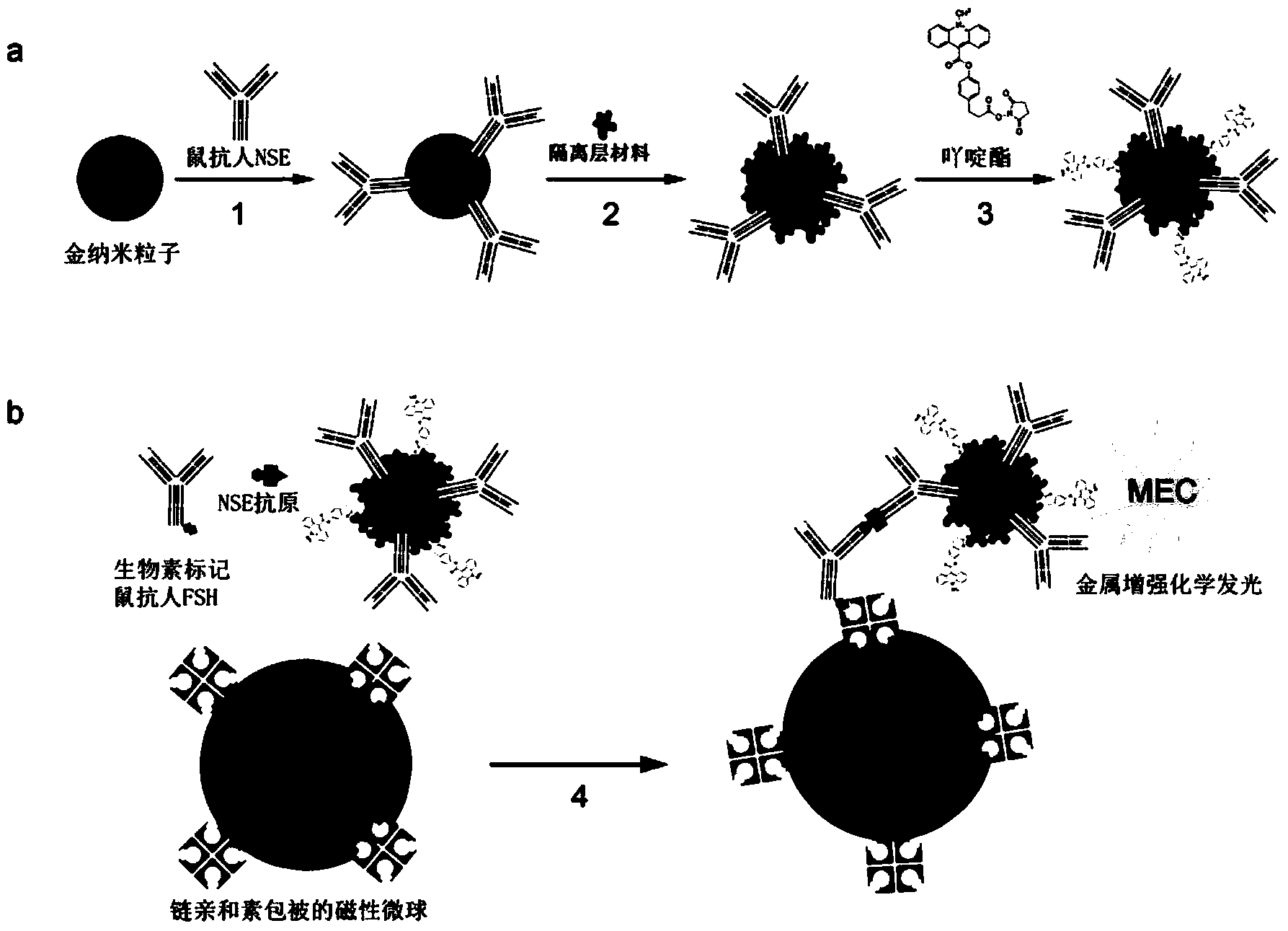
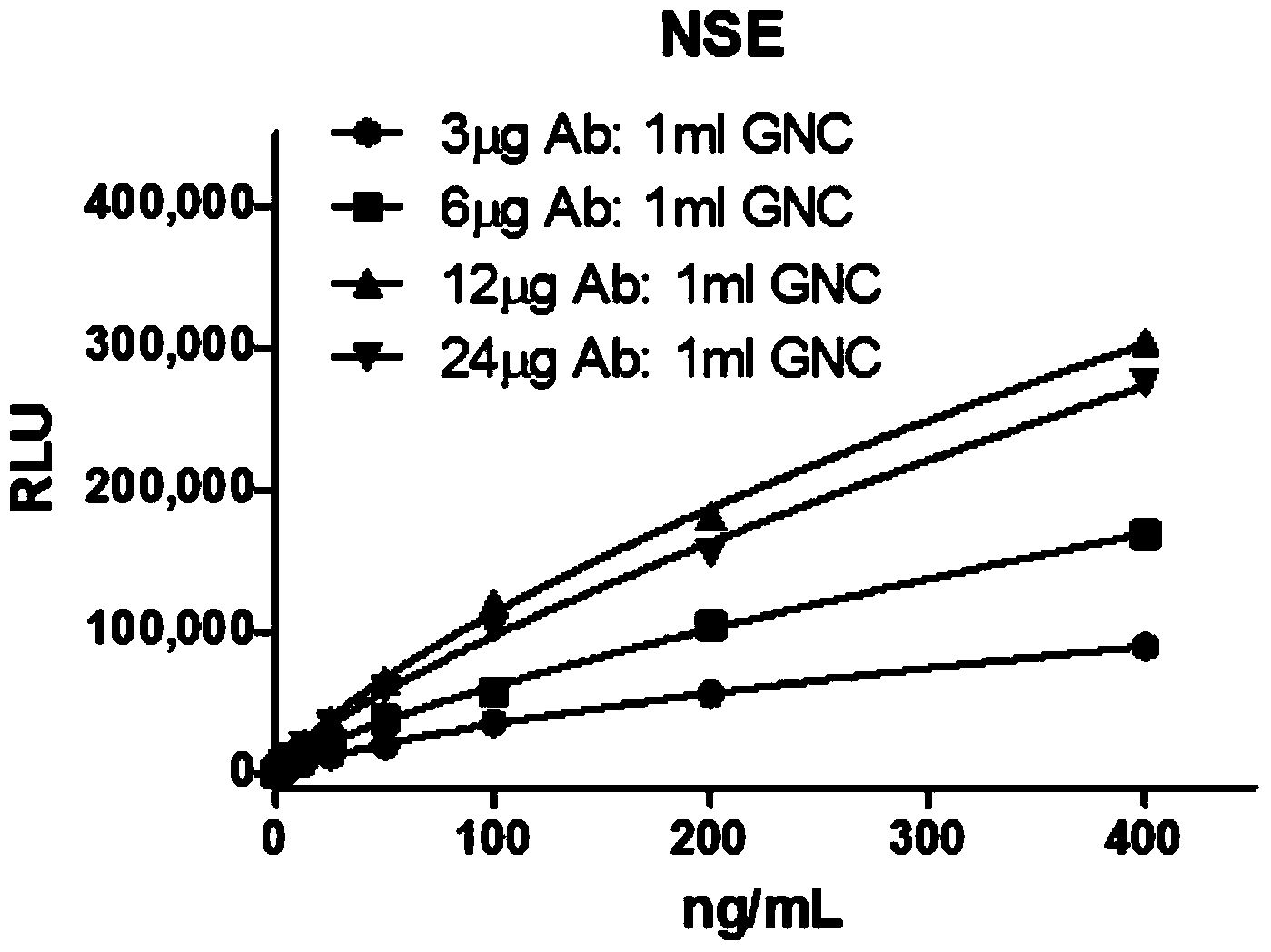
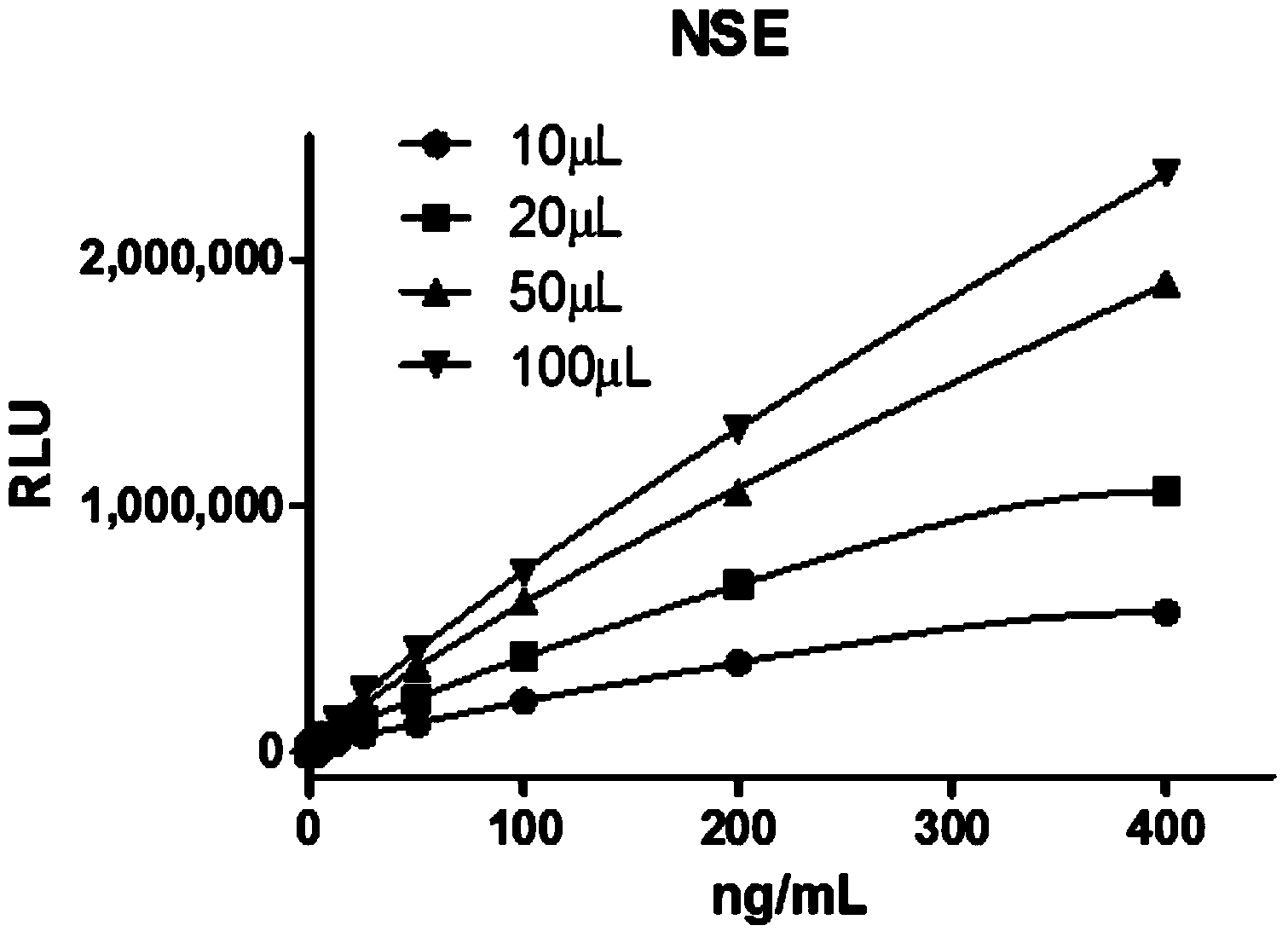
Dual-enhanced chemiluminescent immunoassay method based on metal enhanced luminescence and nano particle labelled amplification
A technology of metal nanoparticles and metal reinforcement, which is applied in the direction of chemical reaction of materials for analysis, chemiluminescence/bioluminescence, and analysis of materials, which can solve the problems of insufficient sensitivity of detection kits and achieve fast detection speed and high sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Double Antibody Sandwich Method and Comparative Experiment
[0044] 1. Preparation of sensitized gold nanoparticles
[0045] 1.1 Preparation of gold nanoparticles with 20nm surface carboxyl groups:
[0046] A. Prepare 1 mL of 4% chloroauric acid solution with deionized water.
[0047] B. Add 0.5mL of the above chloroauric acid solution into 200mL deionized water, stir well and bring to a boil.
[0048] C. Rapidly inject 3ml of 1% sodium citrate aqueous solution into the boiling solution.
[0049] D. Condensate and reflux for 30 minutes. The color of the suspension will change from dark blue to red as the gold nanoparticles form.
[0050] E. Cool to room temperature.
[0051] F. The particle size of the prepared gold nanoparticles measured under a transmission electron microscope is 13nm. The mass of a single spherical gold nanoparticle was calculated according to the particle size and density, and the particle concentration in the nano-gold solution was...
Embodiment 2
[0090] Embodiment 2: competition method and comparative experiment
[0091] 1. Use silicon dioxide to form an isolation layer on the surface of gold nanoparticles and couple E2 (estradiol) monoclonal antibody to the surface of the isolation layer: (the method here is the same as the NES sandwich method)
[0092] 1. Preparation of gold nanoparticles with 120nm surface hydroxyl groups:
[0093] A. Prepare 1 mL of 4% chloroauric acid solution with deionized water.
[0094] B. Add 0.5mL of the above chloroauric acid solution into 200mL deionized water, stir well and bring to a boil.
[0095] C. Rapidly inject 3ml of 1% sodium citrate aqueous solution into the boiling solution.
[0096] D. Condensate and reflux for 30 minutes. The color of the suspension will change from dark blue to red as the gold nanoparticles form.
[0097] E. Cool to room temperature.
[0098] F. The particle size of the prepared gold nanoparticles measured under a transmission electron microscope is 13nm. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com