Ticagrelor crystal form and its preparation method and use
A technology of ticagrelor and crystal, which is applied in the field of crystal form of ticagrelor and its preparation, can solve the problems of poor stability and fluidity, difficult to control, etc., and achieve the effect of good thermal stability, good particle shape and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0079] According to the method in WO01 / 92262A1, ticagrelor amorphous form and crystal form I were prepared, specifically as follows:
[0080] a: Take 1 g of ticagrelor, dissolve it in 10 mL of 50% aqueous ethanol by volume, filter, and lyophilize to obtain amorphous ticagrelor.
[0081] b: Take 2 mg of amorphous ticagrelor, and prepare pure crystal form I in DSC according to the following operation: 35°C to 143°C to 35°C to 148°C to 35°C to 148°C to 35°C. Form I seed crystals were obtained.
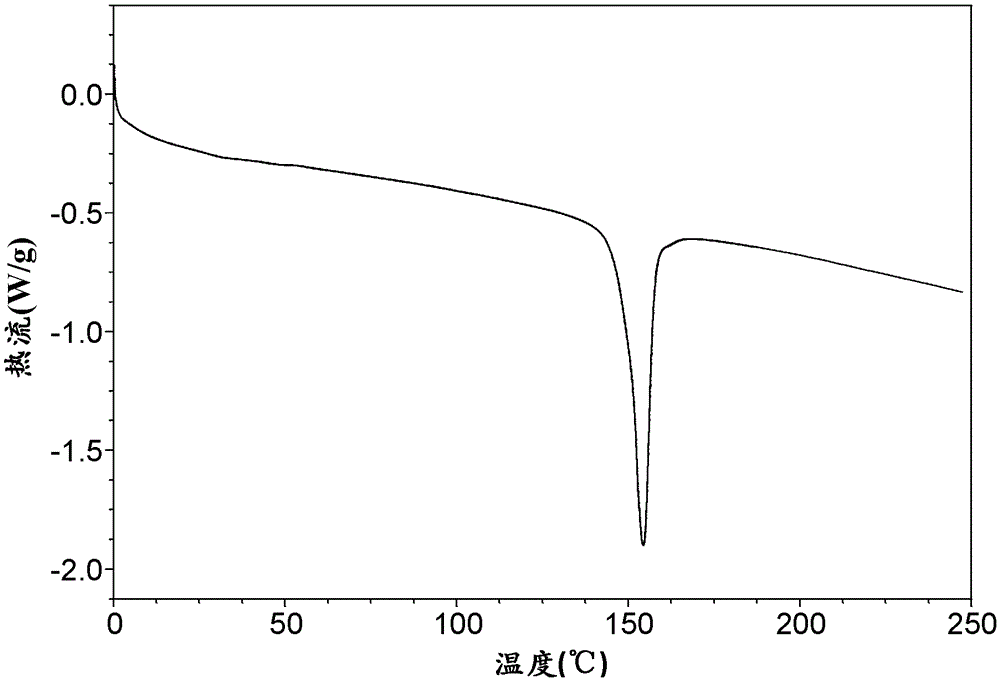
[0082] c: Take 500 mg of amorphous ticagrelor, add 2.5 mL of methanol and 3.65 mL of water, add the seed crystal obtained in b, and crystallize at 30°C to obtain 340 mg of crystal form I. For the DSC spectrum of Form I, see figure 1 , and its melting onset temperature is about 149.7 °C, which is consistent with literature reports.
Embodiment 1
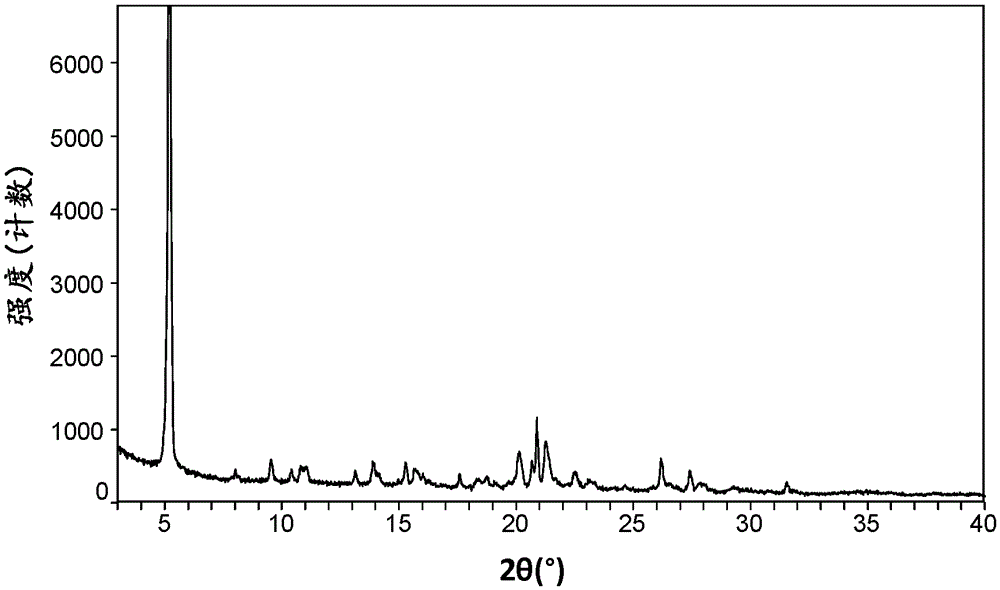
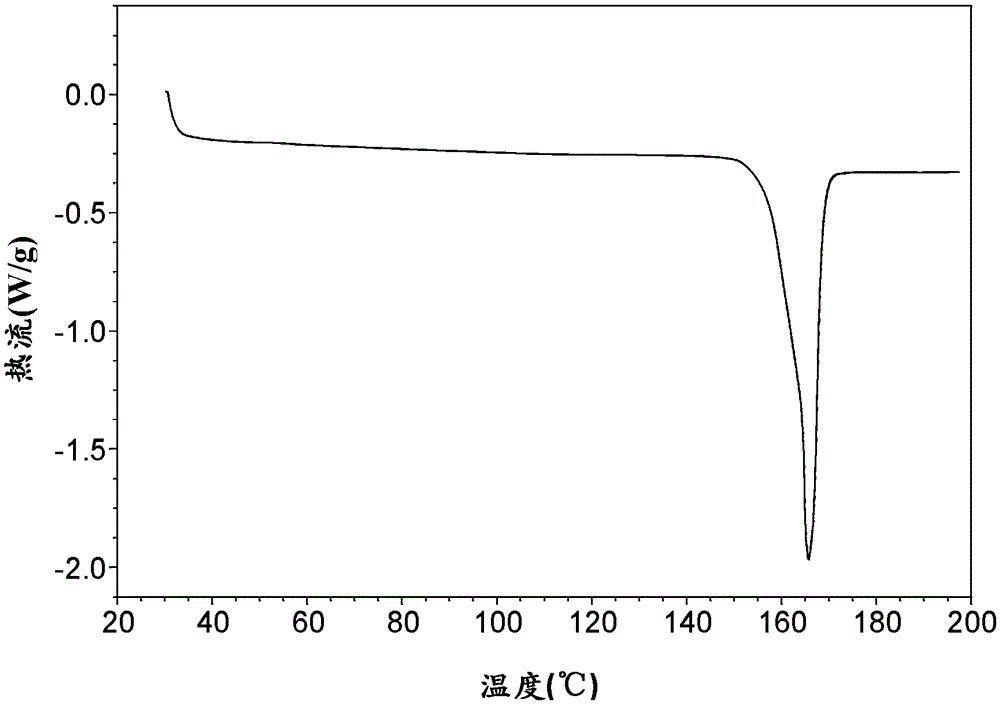
[0084] Take 184mg of amorphous ticagrelor, add it to 38mL (1:1.25) methanol / water mixed solution to obtain a solid suspension, raise the temperature of the solid suspension to 50°C and stir for 0.5h to dissolve completely, keep it for 2 hours, and then add 5 Cool down to 5°C at a cooling rate of °C / min, filter the resulting magma, wash with water, and dry the filter cake in a vacuum oven at room temperature 25°C for 2 hours to obtain white anhydrous Form V (yield 85%), its XRD pattern is shown in figure 2 , see the DSC spectrum image 3 , PLM map see Figure 4 , the onset temperature of its melting is 163°C, and the peak temperature is 166°C.
Embodiment 2
[0086] Take 240 mg of amorphous ticagrelor, add 72 mL of isopropyl ether solution to obtain a solid suspension, stir the solid suspension at 25 ° C for 3 hours, filter the obtained crystal slurry, wash with isopropyl ether, and place the filter cake in Drying in a vacuum oven at 40°C for 2 hours gave white crystals (yield greater than 95%). The starting temperature of melting of this product was 154°C, and the peak value was 157°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com