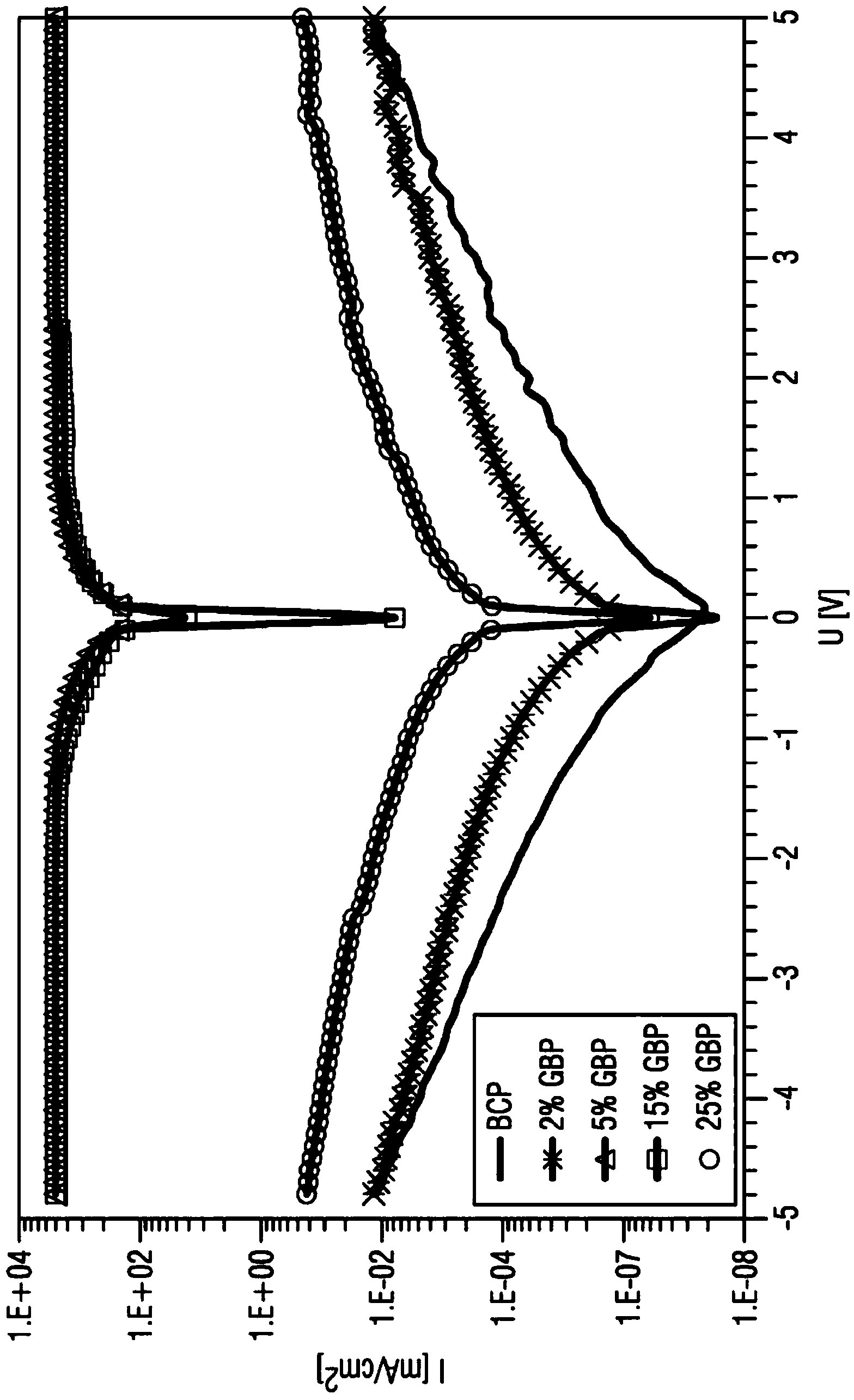
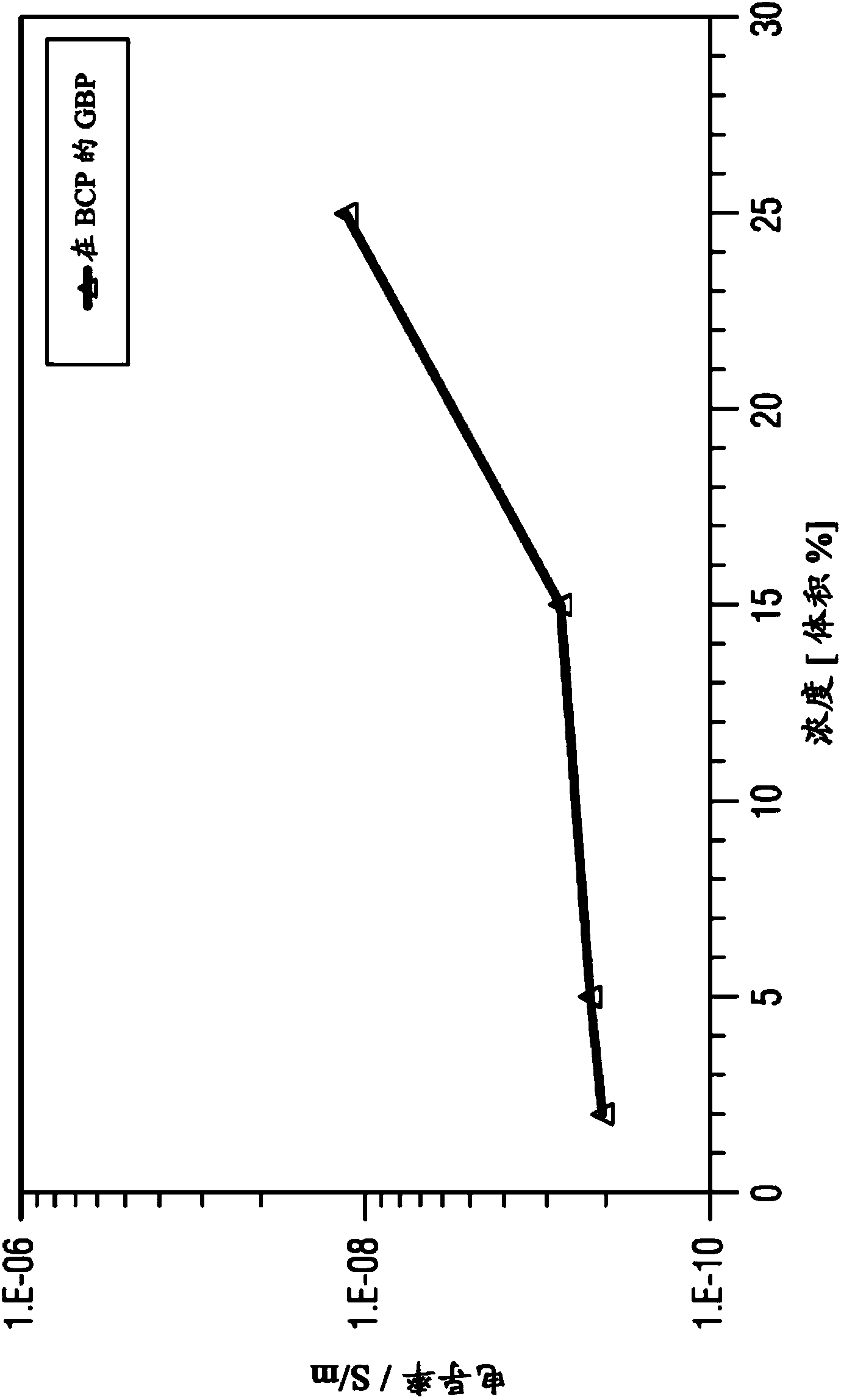
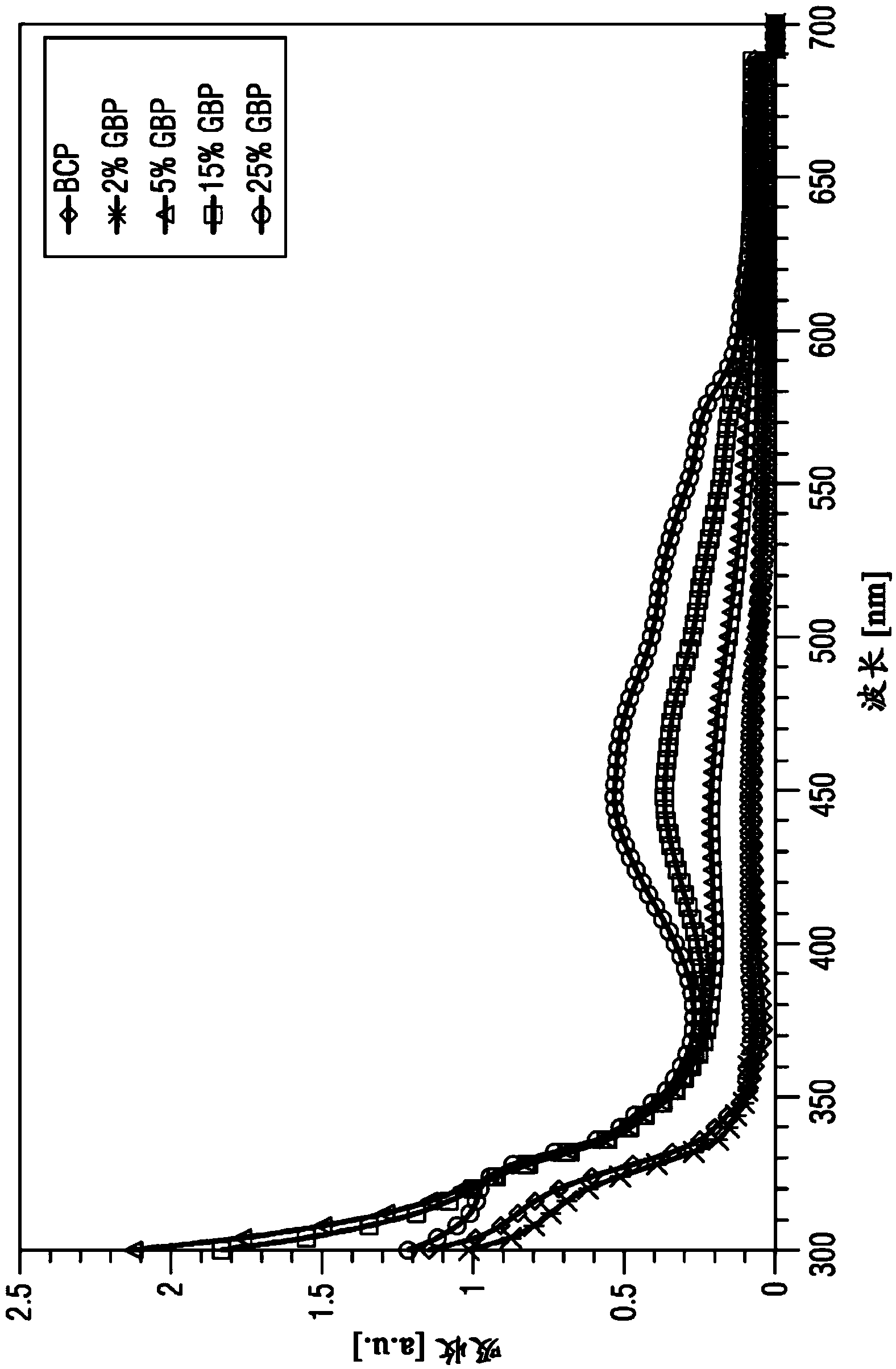
Organic electronic components having organic superdonors having at least two coupled carbene groups and use thereof as an n-type dopants
A technology of organic electronic components and dopants, applied in electrical components, semiconductor devices, electric solid devices, etc., can solve the problems of irreversible separation, limiting component life, dissociation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0077] I. Synthesis
[0078] I.A) Two-step synthesis of 2,2',6,6'-tetraphenyl-4,4'-dipyran (GBP)
[0079] GBP is synthesized in two stages.
[0080] I.A-1) Synthesis of perchloric acid diphenylpyrrolium salt (diphenylpyrylium perchlorate)
[0081] 30g of acetophenone (0.25mol) was dissolved in 250g of ethyl orthoacetate (1.68mol) and boiled. Then 18 g of 70% perchloric acid (0.13 mol) was slowly added dropwise to the solution. The temperature was lowered to 80 °C and the reaction was continued overnight. After cooling, the precipitate formed was filtered off with suction and washed with diethyl ether. This gave a reaction product of bisphenylpyrrolium perchlorate having a melting point of 222° C. according to the following reaction formula.
[0082]
[0083] I.A-2) Synthesis of 2,2',6,6'-tetraphenyl-4,4'-dipyran (GBP)
[0084] Heat 0.2 mol of bisphenylpyrrolium perchlorate in pyridine and 0.01 mol of triphenylphosphine under reflux for 3 hours. After cooling, the pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com