5alpha-androstane-3beta,5,6beta-triol injection and preparing method thereof
The technology of a triol injection and androstatriol, which is applied in the field of medicine, can solve problems such as kidney side effects, and achieve the effects of improving solubility and increasing surface area.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
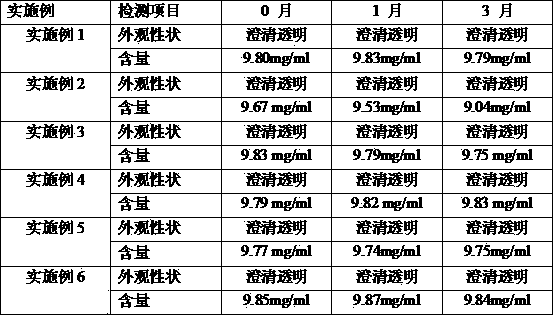
Embodiment 1
[0029] Weigh about 50mg of soybean lecithin, put it in a 50ml round bottom flask, add about 150mg of sodium deoxycholate, and add 7.5ml of ethanol solution (to make the bile salt concentration about 20mg / ml), and ultrasonically make it completely dissolved to form a blank micellar solution. Accurately weigh 20 mg of androstatriol, place it in a round bottom flask, and ultrasonically disperse it evenly. Then use a rotary evaporator to remove ethanol until there is no alcohol smell, make it form a transparent film, disperse it with 2ml of water for injection, and filter through a 0.22 μm microporous membrane to obtain androstatriol mixed micellar injection. The particle size was determined to be about 10-20 nm with a NANO ZS-90 laser particle size analyzer (Malvern, UK).
Embodiment 2
[0031] Weigh about 66mg of egg yolk lecithin, put it in a 50ml round bottom flask, add about 133mg of sodium hyocholate, and add 7.0ml of methanol solution (to make the bile salt concentration about 20mg / ml), and ultrasonically make it completely dissolved to form a blank micellar solution. Accurately weigh 20 mg of androstatriol, place it in a round bottom flask, and ultrasonically disperse it evenly. Then use a rotary evaporator to remove ethanol until there is no alcohol smell, make it form a transparent film, disperse it with 2ml of water for injection, and filter through a 0.22 μm microporous membrane to obtain androstatriol mixed micellar injection.
Embodiment 3
[0033] Weigh about 66mg of soybean lecithin, put it in a 50ml round bottom flask, add about 133mg of sodium glycocholate, and add 7.0ml of chloroform:methanol 1:1 solution (make the bile salt concentration about 20mg / ml) , and ultrasonically dissolve it completely to form a blank micellar solution. Accurately weigh 20 mg of androstatriol, place it in a round bottom flask, and ultrasonically disperse it evenly. Then use a rotary evaporator to remove ethanol until there is no alcohol smell, make it form a transparent film, disperse it with 2ml of water for injection, and filter through a 0.22 μm microporous membrane to obtain androstatriol mixed micellar injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Average concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com