Polyimide adhesive and preparation method thereof
A polyimide and adhesive technology, applied in the direction of adhesives, etc., can solve the problems of limited application, low peel strength, and low adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0066] The present invention provides a kind of preparation method of polyimide adhesive described in above-mentioned technical scheme, comprises the following steps:
[0067] Mixing a diamine having a structure shown in formula II and a first organic solvent to obtain a diamine solution;
[0068] Polymerizing the diamine solution and the dianhydride having the structure shown in formula III to obtain the first polymer intermediate;
[0069] polymerizing the first polymer intermediate with a diamine having a structure shown in formula IV to obtain a second polymer intermediate;
[0070] The second polymer intermediate, the second organic solvent and the diamine having the structure shown in formula V are polymerized to obtain a polyamic acid solution;
[0071] Coating the polyamic acid solution, removing the first organic solvent and the second organic solvent, and then imidizing to obtain a polyimide adhesive;
[0072] h 2 N-R 2 -NH 2 Formula II; h 2 N-R 3 -NH 2 For...
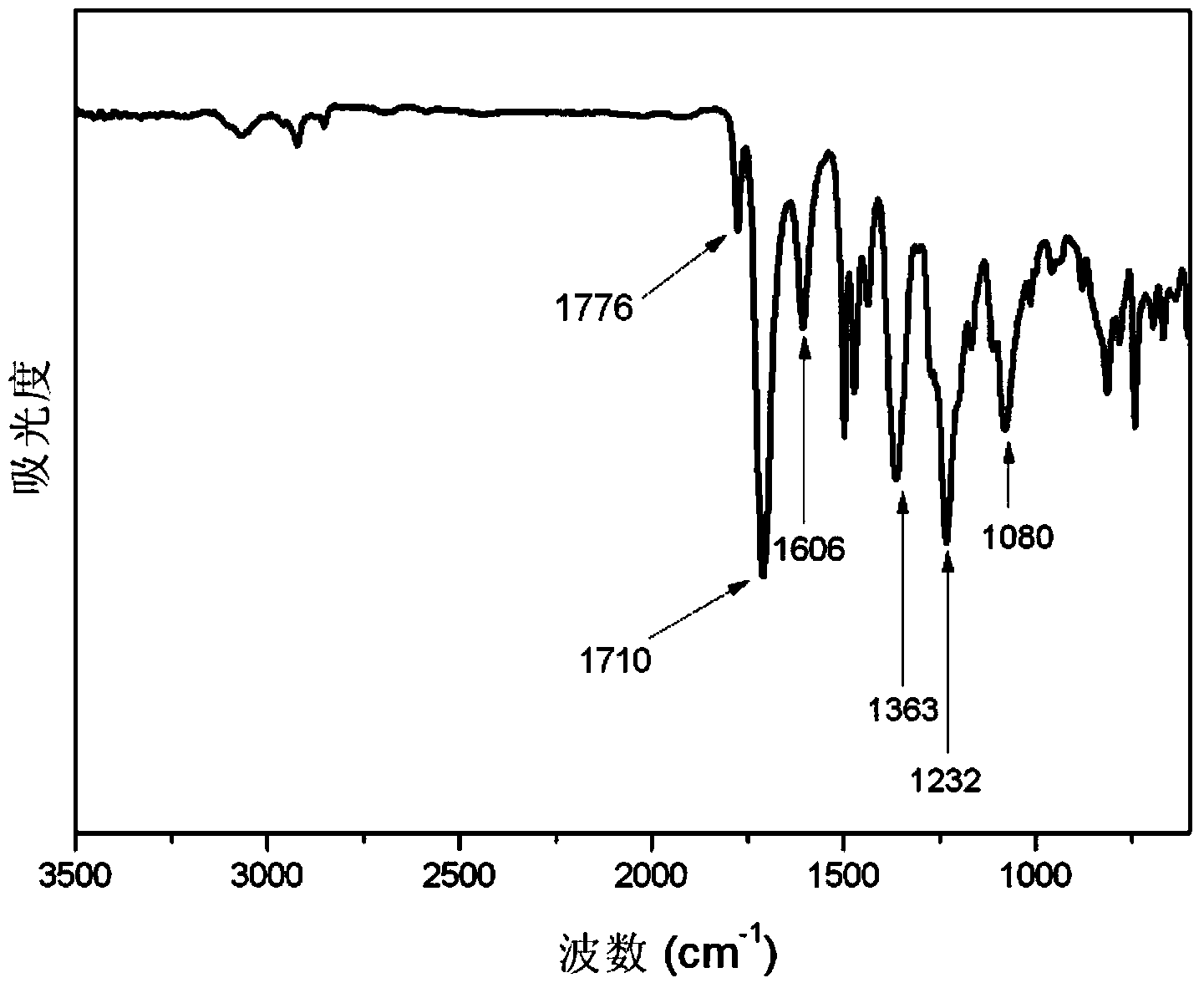
Embodiment 1
[0097] Add 2,5-bis(4-aminophenyl)pyridine (7.84g, 0.03mol) into 140g of N-methylpyrrolidone, stir and dissolve to obtain 2,5-bis(4-aminophenyl)pyridine solution ;
[0098] Add 3,3',4,4'-diphenyl ether tetracarboxylic dianhydride (31.02g, 0.10mol) to the 2,5-bis(4-aminophenyl)pyridine solution, stir and polymerize at 15°C Reaction 4h, obtains the first polymer intermediate;
[0099] Add 3,4'-diaminodiphenyl ether (12.01g, 0.06mol) to the first polymer intermediate, stir, and carry out polymerization reaction at 15°C for 6h to obtain the second polymer intermediate;
[0100] Add 70g of tetrahydrofuran to the second polymer intermediate, then add 1,3-diaminopropylhexamethyldisiloxane (2.48g, 0.01mol), stir, and carry out polymerization reaction at 15°C for 8h to obtain Polyamic acid solution;
[0101] The polyamic acid solution was salivated on the surface of the stainless steel plate, placed in an oven and dried at 80°C for 180 minutes; then, slowly heated to 300°C in the ove...
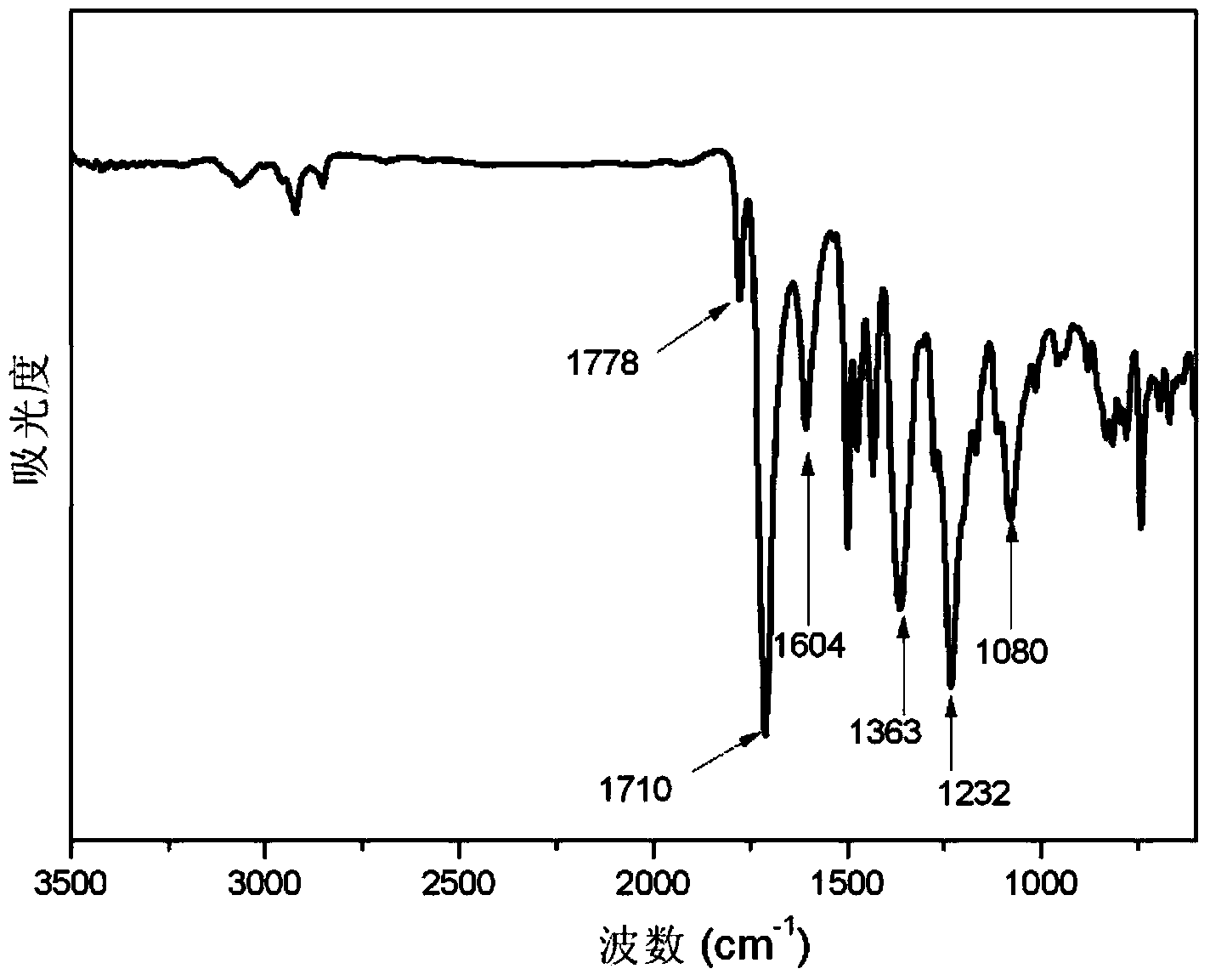
Embodiment 2
[0107] Add 2,5-bis(4-aminophenyl)pyrimidine (7.81g, 0.03mol) into 140g of N-methylpyrrolidone, stir and dissolve to obtain 2,5-bis(4-aminophenyl)pyrimidine solution ;
[0108] Add 3,3',4,4'-diphenyl ether tetracarboxylic dianhydride (31.02g, 0.10mol) to the 2,5-bis(4-aminophenyl)pyrimidine solution, stir and polymerize at 15°C Reaction 4h, obtains the first polymer intermediate;
[0109] 1,3-bis(4-aminophenoxy)benzene (17.54 g, 0.06 mol) was added to the first polymer intermediate, stirred, and polymerized at 15°C for 6 hours to obtain the second polymer intermediate;
[0110] Add 70g of tetrahydrofuran to the second polymer intermediate, then add 1,3-diaminopropylhexamethyldisiloxane (2.48g, 0.01mol), stir, and carry out polymerization reaction at 15°C for 8h to obtain Polyamic acid solution;
[0111] The polyamic acid solution was salivated on the surface of the stainless steel plate, placed in an oven and dried at 80°C for 180 minutes; then, slowly heated to 300°C in the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Peel strength | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com