Respiratory syncytial virus virus-like particle vaccine as well as preparation method and application thereof
A technology for syncytial virus and respiratory tract, which is applied in the field of immune prevention of respiratory syncytial virus infection in humans and susceptible animals, and can solve problems such as the imbalance of cytokine ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] [Example 1] Construction and detection of respiratory syncytial virus virus-like particles (VLPs)
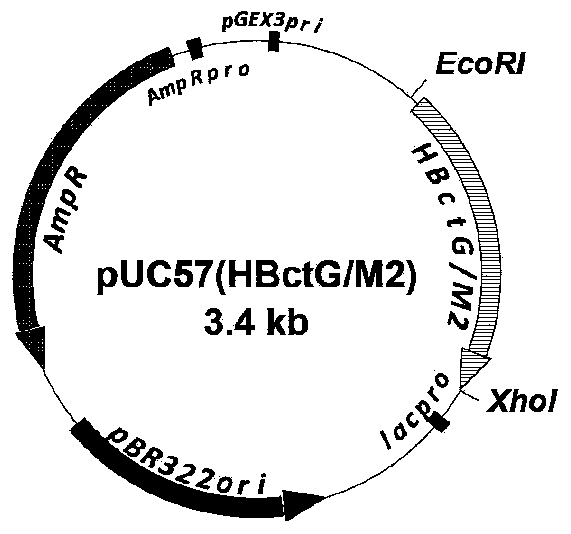
[0038] 1.1. Plasmid pUC57 (HBc-tG / M2 82-90 ) structure diagram
[0039] Plasmid pUC57-HBc-tG / M2 82-90 It is a plasmid vector comprising the chimeric coding gene sequence of the conserved antigen region of RSV G protein and the CTL epitope of M2 through codon optimization design and screening of the present invention. The protein encoded by the gene sequence is the antigen fragment (G144-204aa) (Plotnicky-Gilquin et al., 1999) of the G protein conserved B cell epitope encoded by RSV inserted between Asp78 and Pro80 of the HBc peptide, and the CTL epitope 82-90aa of RSV-encoded M2 protein (M2 82-90 ) fused to the C-terminus of the truncated protein HBc1-144aa, M2 82-90 The epitope can effectively activate the body's CD8 + T lymphocyte immune response (Srikiatkhachorn and Braciale, 1997). It is synthesized by a commercial company entrusted by the inventor, and the inte...
Embodiment 2
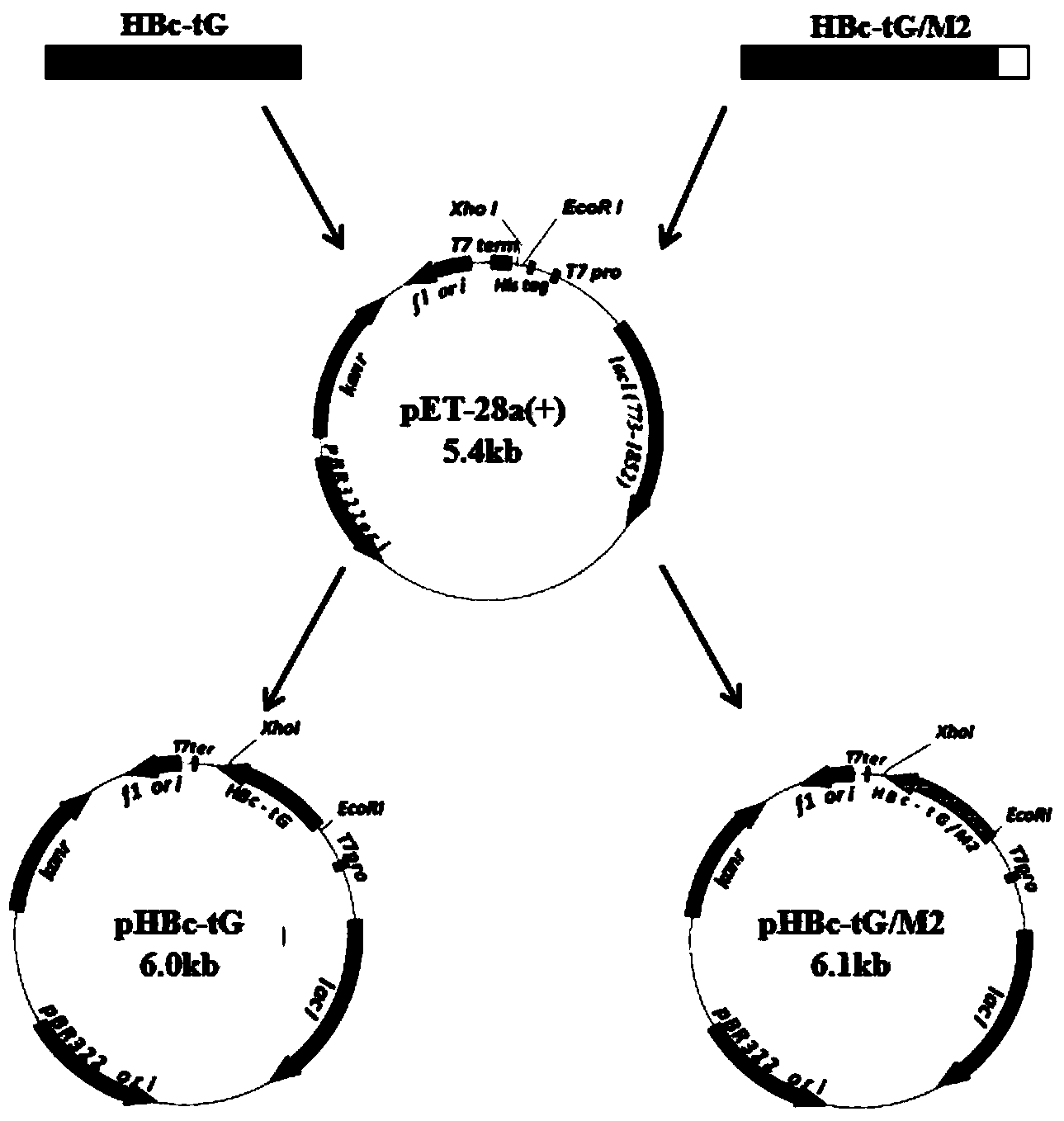
[0163] [Example 2] Large-scale preparation and verification of respiratory syncytial virus virus-like particles (VLPs) vaccine
[0164] 2.1. Large-scale preparation of respiratory syncytial virus-like particle (VLPs) vaccine
[0165] 1) Recombinant antigenic protein HBc-tG and HBc-tG / M2 82-90 a large number of expressions
[0166] (1) Take the frozen engineered bacteria out of the -80°C refrigerator, thaw, transfer 0.1ml of the bacteria liquid to 5ml of LB medium (containing kanamycin and chloramphenicol), and rotate at 220rpm at 37°C overnight.
[0167] (2) Inoculate the overnight culture into 1 LLB medium containing kanamycin and chloramphenicol at a ratio of 1:100, culture at 37°C with shaking at 220rpm. To be cultured to the logarithmic growth phase (bacterial solution OD 600 Value reaches 0.6), add IPTG to the final concentration of 0.2mM, 37°C, 220rpm rotating culture for 5 hours, induce protein expression.
[0168] (3) Transfer the induced culture to a 250ml centrif...
Embodiment 3
[0214] [Example 3] Functional detection of respiratory syncytial virus virus-like particles (VLPs) vaccine
[0215] Experimental protocol
[0216] Take 40 female BALB / c mice aged 5-6 weeks, and randomly divide them into 4 groups of A, B, C, and D, with 10 mice in each group. Before vaccination, blood is collected from the tail of the mice, and the serum is separated for antibody detection as negative control serum. Immunization was designed according to the following: Group A was injected with UV-inactivated RSV (1×10 5 TCID50); group B was injected with HBc-tG (VLPs), dose 20 μg / only; group C was injected with HBc-tG / M2 82-90 (VLPs), the dose is 20 μg / only. Vaccines were immunized by intramuscular injection, and each vaccine was immunized three times with an interval of three weeks; group D was the control group, which was injected with PBS by the same route and at intervals. Two weeks after immunization, blood was collected by tail docking and serum was separated. After...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com