A kind of varicella-zoster virus gb-ge-gh-gl fusion protein, genetic engineering subunit vaccine and preparation method
A technology of gb-ge-gh-gl, herpes zoster virus, applied in the field of biomedicine, can solve the problems of less than 80% protection rate of live VZV vaccine, increased possibility of virus reactivation infection, dangerous systemic infection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of gB-gE-gH-gL fusion protein
[0030]Prokaryotic expression of step 1 gB-gE-gH-gL fusion protein
[0031] The blister liquid collected from the skin blisters of chickenpox patients was inoculated onto MRC-5 cells, and the VZV virus was isolated and cultured. After the cells became lesions, the cells were collected, and the DNA was extracted by the phenol: chloroform: isoamyl alcohol method, and used as PCR amplification. Added templates;
[0032] According to the sequence of VZV Dumas (X04370.1) strain in Genebank, amino acid positions 136-285 of VZV gB protein, amino acid positions 37-161 of gE protein, amino acid positions 18-168 of gH protein, and amino acid positions of gL protein were respectively designed The nested PCR primers at positions 23-160 are connected with GGGGS between each two proteins, and NdeI and NocI restriction sites are reserved at both ends of the final gB-gE-gH-gL fusion gene;
[0033] Insert the gB-gE-gH-gL fusion gene...
Embodiment 2
[0036] The immune effect detection of embodiment 2 subunit vaccine
[0037] Use the BCA method to detect the protein concentration of the purified gB-gE-gH-gL fusion protein, dilute it to 0.5mg / mL with PBS, filter it aseptically, and set aside; carry out the sterility test according to the current "Chinese Pharmacopoeia", and use the Limulus reagent method Endotoxin testing is carried out, and the endotoxin content is not higher than 100EU / mL before use;
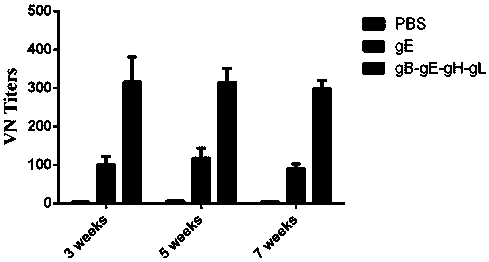
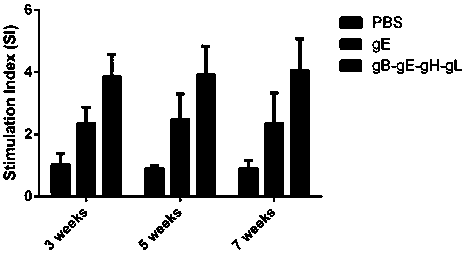
[0038] Mix gB-gE-gH-gL fusion protein with an equal volume of aluminum hydroxide adjuvant or Freund's complete adjuvant 1:1, fully emulsify, and immunize 6-week-old BALB / c mice intraperitoneally, 25 μg / mouse Mice; 2 weeks after the initial immunization, booster immunization once, the dose was the same as the first time; at the same time, the same dose of recombinant VZV gE protein and PBS was inoculated as a control.
[0039] Three mice were sacrificed at the 3rd, 5th, and 7th weeks after inoculation, and the neutralizing a...
Embodiment 3
[0044] Embodiment 3 challenge protection test
[0045] A VZV strain that can adapt to guinea pig cells was cultivated in vitro, and then the peripheral blood lymphocytes of guinea pigs were infected with the virus strain in vitro, and then the guinea pig lymphocytes infected with VZV were reinfused into guinea pigs, and 28 days later, the intestinal ganglion of guinea pigs and establish a latent infection in the dorsal root ganglia. Utilize this model to carry out challenge protection test, divide 8 weeks old female Hartley guinea pigs 12 into two groups, every group 6, the first group is immune group, carries out secondary immunization with VZV genetic engineering subunit vaccine prepared by the present invention ( The second immunization was carried out 14 days after the first immunization, each time 100 μg per mouse, subcutaneous injection), the second group was the normal saline control group, and the same volume of sterile normal saline was injected. 28 days after the se...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com