Preparation method of glp-1 or its analogue and antibody fc fragment fusion protein
A fusion protein, GLP-1 technology, applied in the biological field, can solve the problems of reducing the degree of degradation of the N-terminal of the GLP-1-Fc molecule, decreasing the yield, and being uneconomical
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
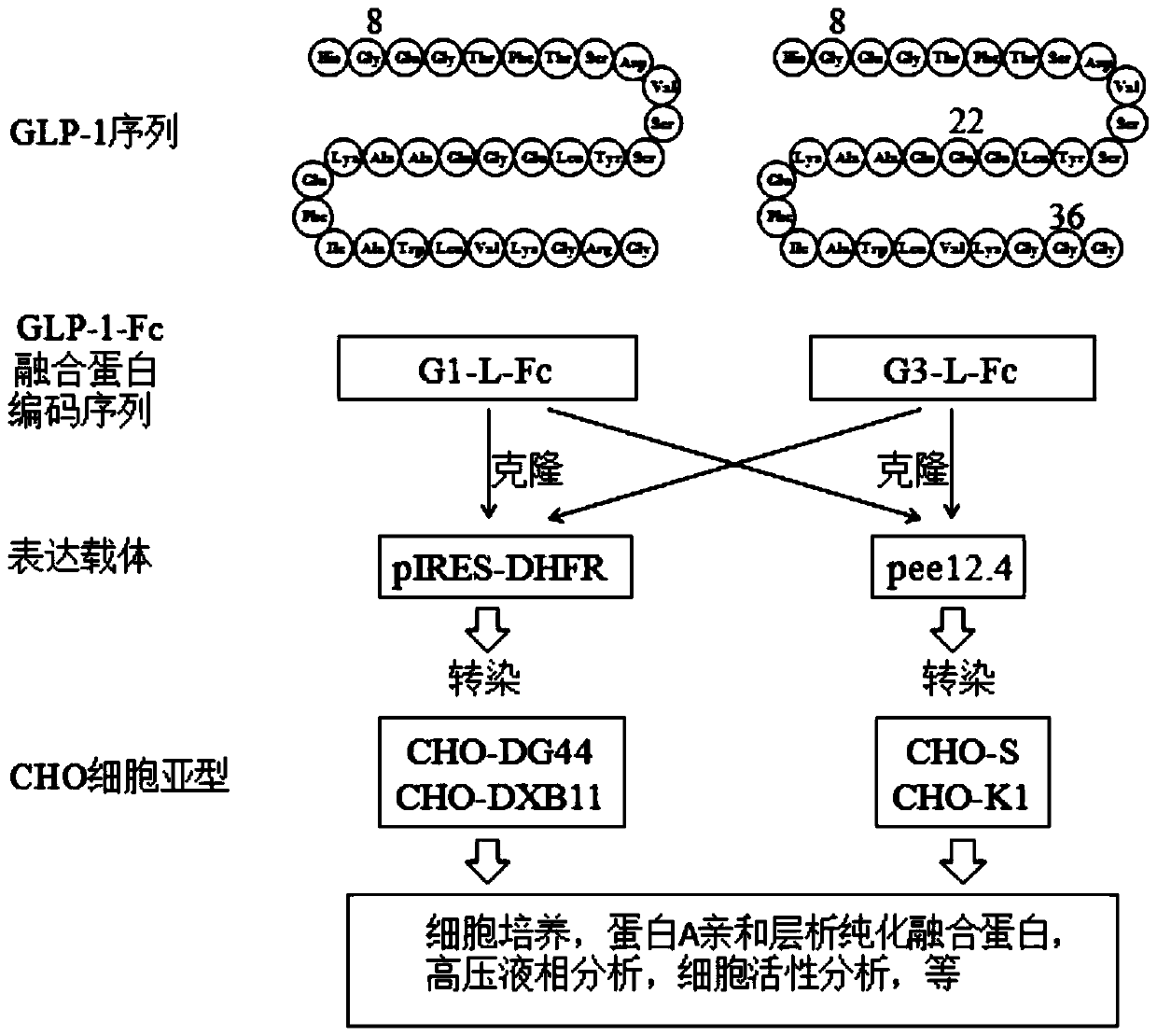
[0043] The invention provides a method for preparing a fusion protein of GLP-1 or its analogue and an antibody Fc fragment, comprising the following steps:
[0044] 1) Cloning the coding sequence of GLP-1 or its analogue and antibody Fc fragment fusion protein (GLP-1-Fc) into an expression vector;
[0045] 2) Transfect the expression vector into CHO-DXB11 cells, cultivate and screen positive cell lines;
[0046] 3) After using the cells obtained in step 2 to express and purify, the fusion protein is obtained.
[0047] In the preparation method of the fusion protein of GLP-1 or its analogue and antibody Fc fragment provided by the present invention, the fusion protein of GLP-1 or its analogue and antibody Fc fragment is composed of recombinant human glucagon-like peptide-1 (GLP- 1) or its analogues are formed by linking a linking peptide with an antibody Fc fragment that can prolong the half-life in the human body.
[0048] In the fusion protein of GLP-1 or its analogue and a...
Embodiment 1
[0081] Coding sequence of recombinant human GLP-1-Fc fusion protein
[0082] 1. Amino acid composition of GLP-1
[0083] Natural GLP-1 and any modified GLP-1 molecules can form fusion proteins by linking different flexible linking peptides with different Fc fragments, such as IgG1, IgG2, IgG3, IgG4 and modified ones. These fusion proteins Both have biological activity and long-term effect.
[0084] The amino acid sequence of native GLP-1 is as follows:
[0085] HAEGTFTSDVSSYLEGQAAKEFIAWLVKGRG (SEQ ID No: 14).
[0086] The fusion protein molecule of the present invention is a variant modified on the basis of the original GLP-1 molecule, specifically two variants modified on the basis of the 31 amino acids of the original GLP-1 molecule. Its name and molecule The structures are:
[0087] Molecular name: G1 (1 amino acid modification in the 31 amino acid GLP-1 molecule)
[0088] Modification: Gly8-GLP-1 (7-37), the first amino acid number of the original GLP-1 is 7 (position...
Embodiment 2
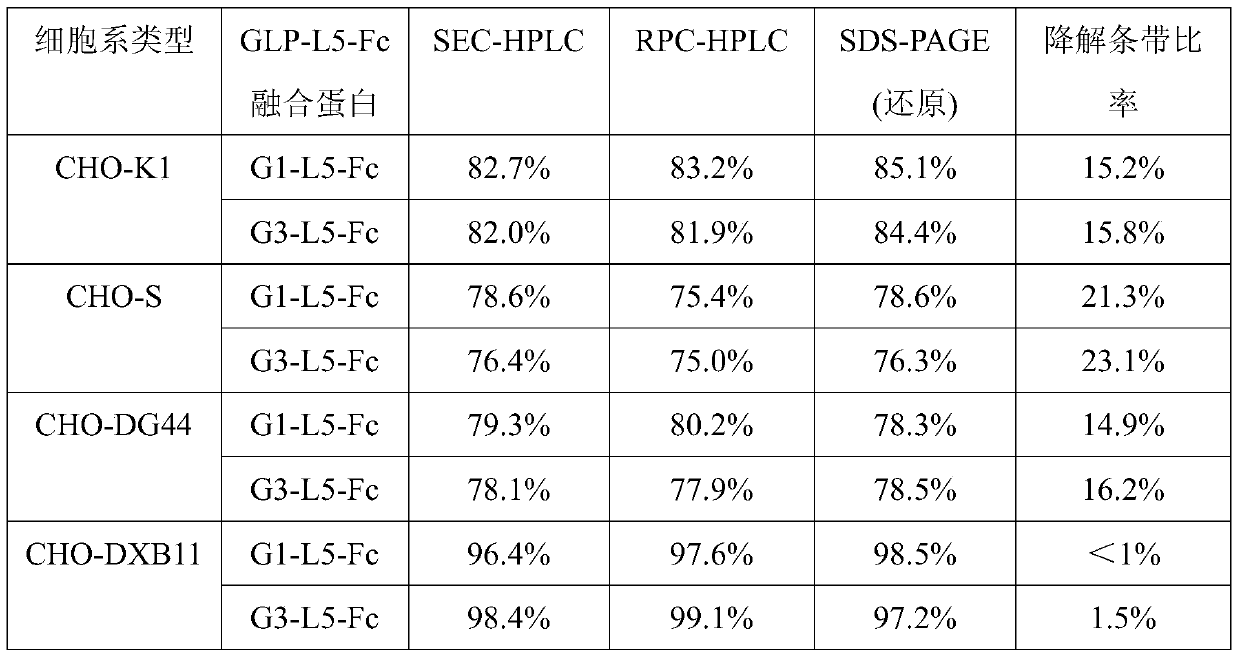
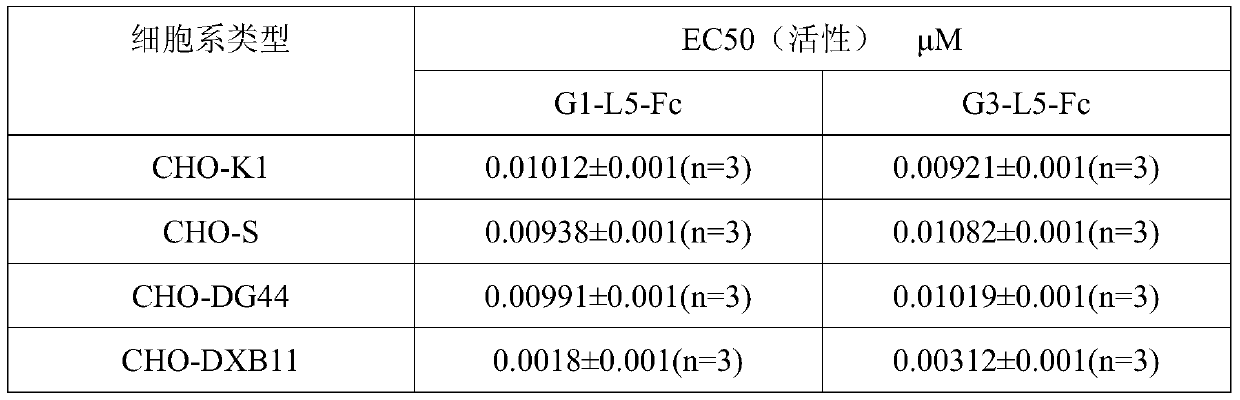
[0114] Expression of GLP-1-Fc fusion protein in CHO-DXB11 and CHO-DG44 cells:
[0115] Two kinds of proteins (G1-L5-Fc and G3-L5-Fc) coding sequence SEQ ID No: 12 and SEQ ID No :13 was cloned into the expression vector sequence containing IRES-DHFR, and the expression vector expressed GLP-1-Fc under the drive of CMV promoter.
[0116] The coding sequence of G1-L5-Fc is shown in SEQ ID No: 12: (signal peptide containing 19aa)
[0117] atggagtggtcctgggtgttcctgttctttctgtccgtgaccacaggagtccacagccatggtgaagggacctttaccagtgatgtaagttcttatttggaaggacaagctgccaaggagttcattgcttggctggtgaaaggccgtggaggatccggtggcggttccggcggtggaggatcaggaggcggtggctctggaggtggtggaagtggtggcggcggttcgtctaagtacgggcccccttgccctccttgcccagctcctgaatttgagggcggacccagcgtgttcctgttccccccaaagcccaaggacaccctgatgatcagcagaacccccgaagtgacctgcgtggtggtggacgtgtcccaggaagatcccgaggtgcagttcaattggtacgtggacggcgtggaagtgcacaacgccaagaccaagcccagagaggaacagttcaacagcacctacagagtggtgtccgtgctgaccgtgctgcaccaggattggctgaacggcaaagagtacaagtgcaaggtgtccaacaagggcc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com