Method for preparing chloroethane from chlorination by-product hydrogen chloride
A technology of chlorination reaction and ethyl chloride, which is applied in the preparation of halogenated hydrocarbons, chemical instruments and methods, dehydration of hydroxyl-containing compounds to prepare ethers, etc., can solve the problems of low recycling value, high cost of pollution control, and easy pollution of the environment , to achieve low production consumption, reduce production costs, and eliminate the effects of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
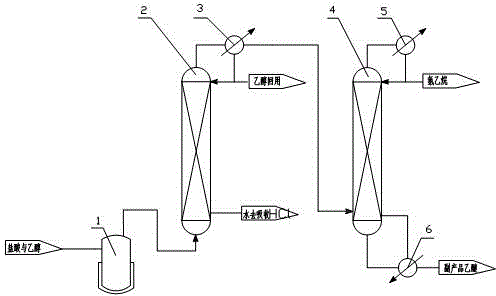
[0014] Example 1: Hydrogen chloride, by-product of chlorination reaction, was prepared by following steps to prepare ethyl chloride
[0015] (1) Reaction: add 30% mass concentration of zinc chloride aqueous solution in reactor 1, zinc chloride is used as a catalyst, and the heating temperature rises to 120 ° C, and the mass concentration of 30% hydrochloric acid and mass concentration of 95% ethanol are molar The ratio of 1:0.70 is passed into the reactor to react;
[0016] (2) Rectification: the reacted gaseous mixture enters the first rectification tower 2, and the water containing 7% HCl flows out and reused from the bottom of the tower, and uses it to absorb the by-product hydrogen chloride of the chlorination reaction; the tower of the first rectification tower 2 The overhead steam is condensed 3 through the first condenser, the condensing temperature is 25 ° C, the reflux ratio is 1.5, the extraction content of 95% ethanol enters the storage tank for reuse, and the non-c...
Embodiment 2
[0017] Embodiment 2: Chlorination reaction by-product hydrogen chloride prepares chloroethane according to the following steps
[0018] (1) Reaction: add 40% aqueous zinc chloride solution by mass concentration in reactor 1, zinc chloride is used as a catalyst, and the heating temperature rises to 135 ° C, and the hydrochloric acid that mass concentration is 33% and the mass concentration of 95% ethanol are molar The ratio of 1:0.75 is passed into the reactor to react;
[0019] (2) Rectification: the reacted gaseous mixture enters the first rectification tower 2, and the water containing 9% HCl flows out and reused from the bottom of the tower, and uses it to absorb the by-product hydrogen chloride of the chlorination reaction; the tower of the first rectification tower 2 The overhead steam is condensed 3 through the first condenser, the condensing temperature is 26 ℃, the reflux ratio is 1.5, the ethanol of the extraction content 95% enters the storage tank for reuse, and the...
Embodiment 3
[0020] Embodiment 3: Chlorination reaction by-product hydrogen chloride prepares chloroethane according to the following steps
[0021] (1) Reaction: adding 50% mass concentration of zinc chloride aqueous solution in reaction kettle 1, zinc chloride is used as a catalyst, the heating temperature rises to 140 ° C, and the mass concentration of 35% hydrochloric acid and 95% mass concentration of ethanol are molar The ratio of 1:0.80 is passed into the reactor to react;
[0022] (2) Rectification: the reacted gaseous mixture enters the first rectification tower 2, and the water containing 11% HCl flows out and reused from the bottom of the tower, and uses it to absorb the by-product hydrogen chloride of the chlorination reaction; the tower of the first rectification tower 2 The top steam is condensed through the first condenser 3, the condensing temperature is 27 ° C, the reflux ratio is 1.5, the extraction content 95% ethanol enters the storage tank for reuse, and the non-conden...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com