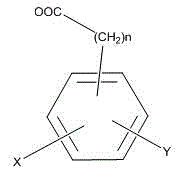
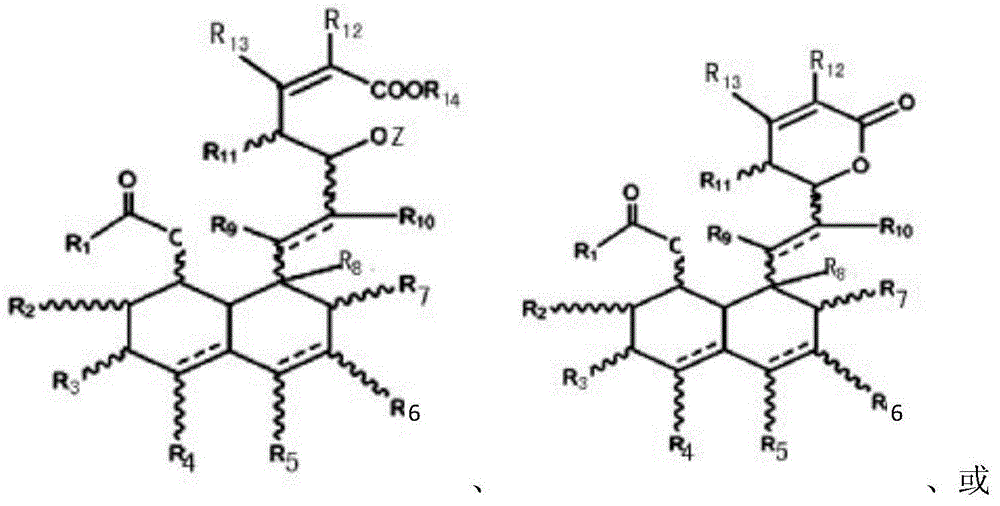
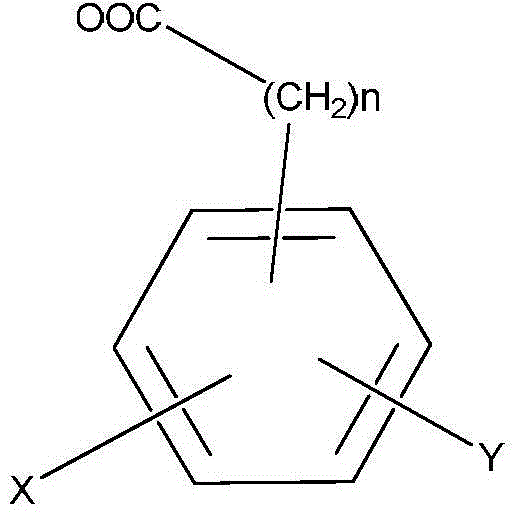
Polysubstituted phenanthrene ring statin lactone dehydrated compounds and application thereof
A technology of compounds and substituents, applied in the field of medicinal chemistry, can solve problems such as carcinogenic toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0203] Embodiment 1 Preparation of pravastatin lactone
[0204] Weigh 5.00g of pravastatin sodium salt, add it to a 250ml eggplant-shaped bottle, add 100ml of dichloromethane and about 10ml of 20-fold diluted hydrochloric acid, acidify, separate, and extract three times with 100ml of dichloromethane. The lower organic phases were combined and dried over anhydrous sodium sulfate. Concentrate and vacuumize with an oil pump to obtain 4.54 g of white powder, which is the crude product of pravastatin carboxylic acid
[0205] Weigh 4.5 g of the crude product of pravastatin carboxylic acid above, add it into a three-necked flask, add 0.05 g of p-dimethylaminopyridine, 50 ml of dichloromethane and a stirring bar. A solution obtained by dissolving 5.0 g of dicyclohexylcarbodiimide in 20 ml of dichloromethane was slowly injected under ice cooling. After the dropwise addition, the ice bath was removed and the reaction was carried out overnight at room temperature. After the completion...
Embodiment 2
[0206] Example 2 Preparation of Compound 001-004
[0207] Add a magnetic stirring bar of appropriate size into a 50ml two-necked reaction flask, add 0.50g of p-toluenesulfonic acid (PBSA) and 1.50g of mevastatin to replace the air and protect it with nitrogen, inject 30ml of tetrahydrofuran and heat the reaction vessel to reflux, After the reaction was completely monitored by thin-layer chromatography, add 5% NaHCO 3 Shake the aqueous solution, separate the lower organic phase, dry over anhydrous sodium sulfate, concentrate, and separate by column chromatography (PE / EA gradient elution) to obtain the mevastatin derivative containing 2-hexenolactone fragment of the present invention (001) 0.76 g.
[0208] Compounds 002, 003 and 004 can be obtained by the same method.
Embodiment 3
[0209] Example 3 Preparation of Compound 005-008
[0210] 0.58 g of mevastatin was dissolved in 15 ml of methanol, 50 mg of 10% Pt / C hydrogenation catalyst was added, pressurized at 1.5 MPa, and reacted overnight at room temperature. After the reaction was complete, the catalyst was filtered off, concentrated and spin-dried to obtain 0.52 g of crude hydromevastatin. The crude product was as in Example 2, and 0.50 g of the crude product of hydromevastatin and 0.20 g of p-toluenesulfonic acid were added. Finally, column chromatography (PE / EA gradient elution) yielded 0.36 g of a hydromevastatin derivative (005) containing a 2-hexenolactone segment.
[0211] Compounds 006, 007 and 008 can be obtained by the same method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com