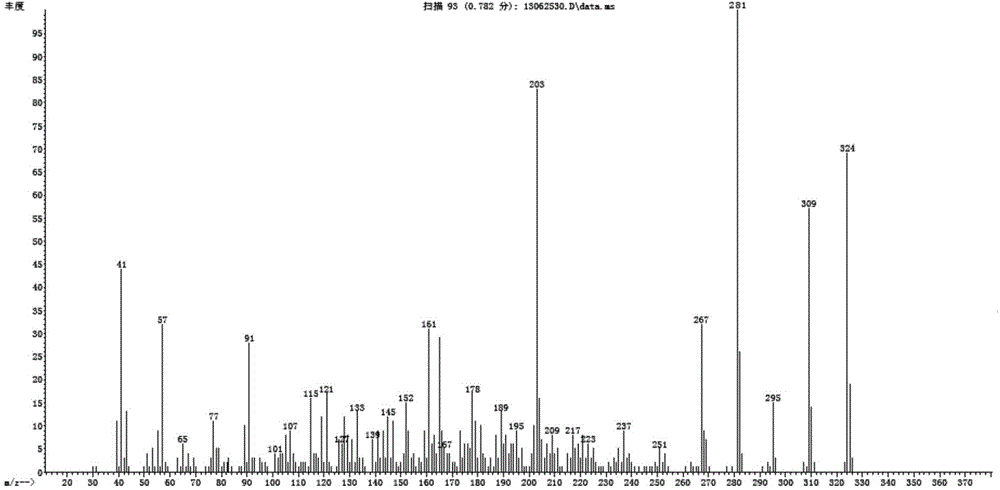
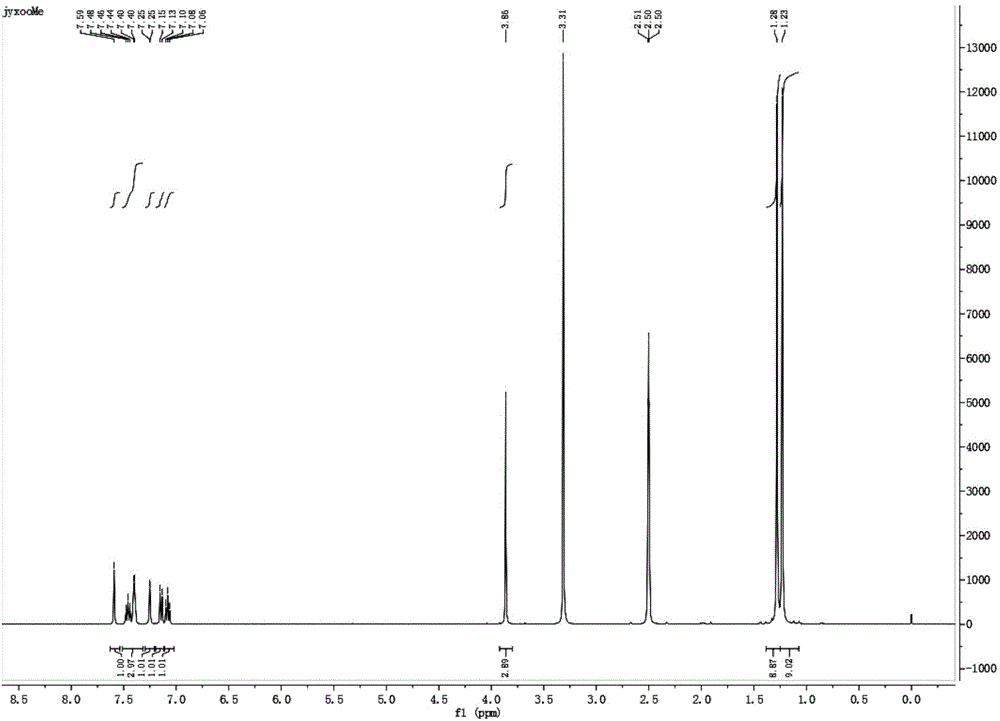
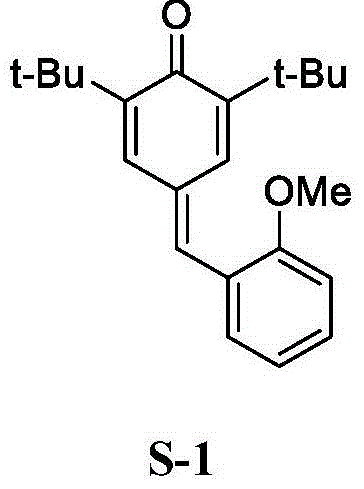
2, 6-di-tert-butyl-4-(2-methoxyphenylmethylene)-2, 5-cyclohexadiene-1-one and preparation method thereof
A technology of methoxybenzylidene and di-tert-butylphenol, which is applied in the field of synthesis of organic compounds, can solve the problems of high operation and equipment requirements, large environmental pollution, high price, etc., to improve the conversion rate of raw materials, reduce the The effect of reaction yield and accelerated formation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1, a kind of 2,6-di-tert-butyl-4-(-2-methoxybenzylidene)-2,5-cyclohexadien-1-one (QM-ph-o-OMe ) preparation method, the following steps are carried out successively:
[0049] A. Preparation of Mannich bases
[0050] Add 41.2g (0.2mol) 2,6-di-tert-butylphenol, 32.7g (0.24mol) o-methoxybenzaldehyde in a 250mL three-necked flask with a condensing water separator and a stirrer, stir and heat to a small Reflux (about 145°C), keep warm after reflux for 20 minutes, slowly drop 20mL (0.2mol) of piperidine, drop it in 3 hours, evaporate the generated water during the reaction, continue to keep warm for 6 hours after dropping The reaction was stopped and monitored by GC during the reaction.
[0051] B. Mannich base removal of secondary amines
[0052] Transfer the above reaction solution into a 250mL single-necked flask, carry out vacuum distillation under the pressure of 150mmHg, heat the flask with an oil bath at a temperature of 120°C, and continue the distillati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com