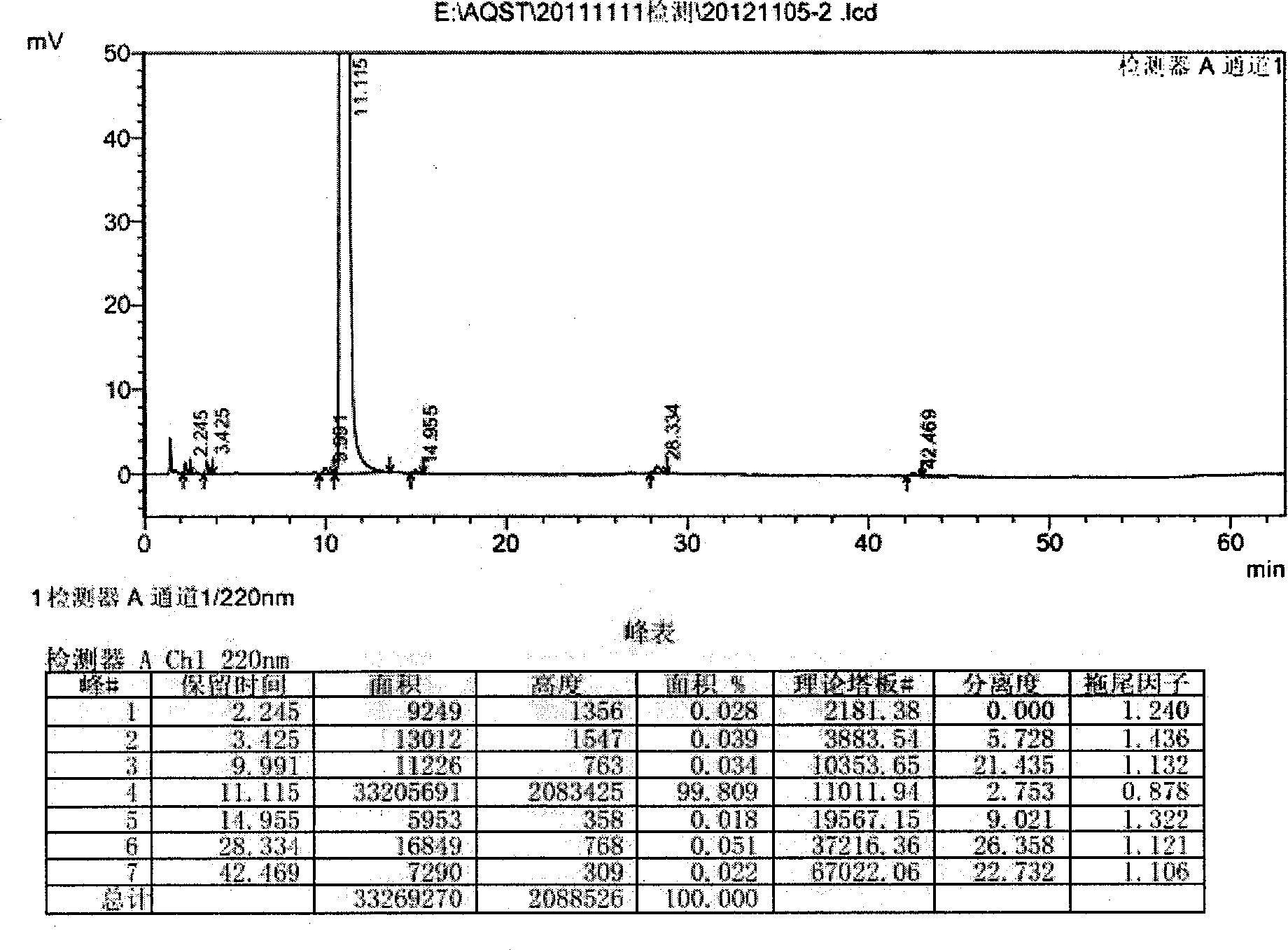
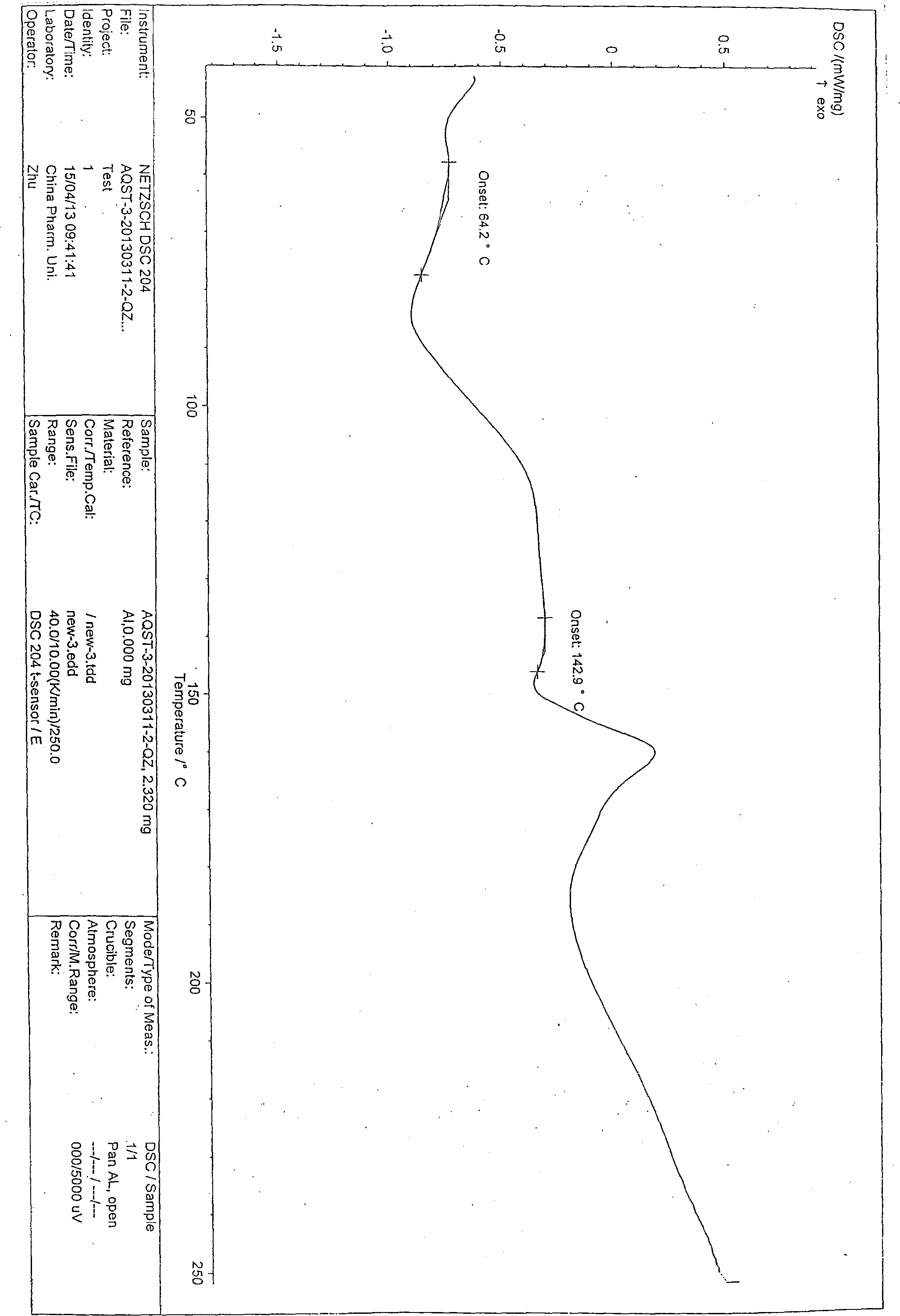
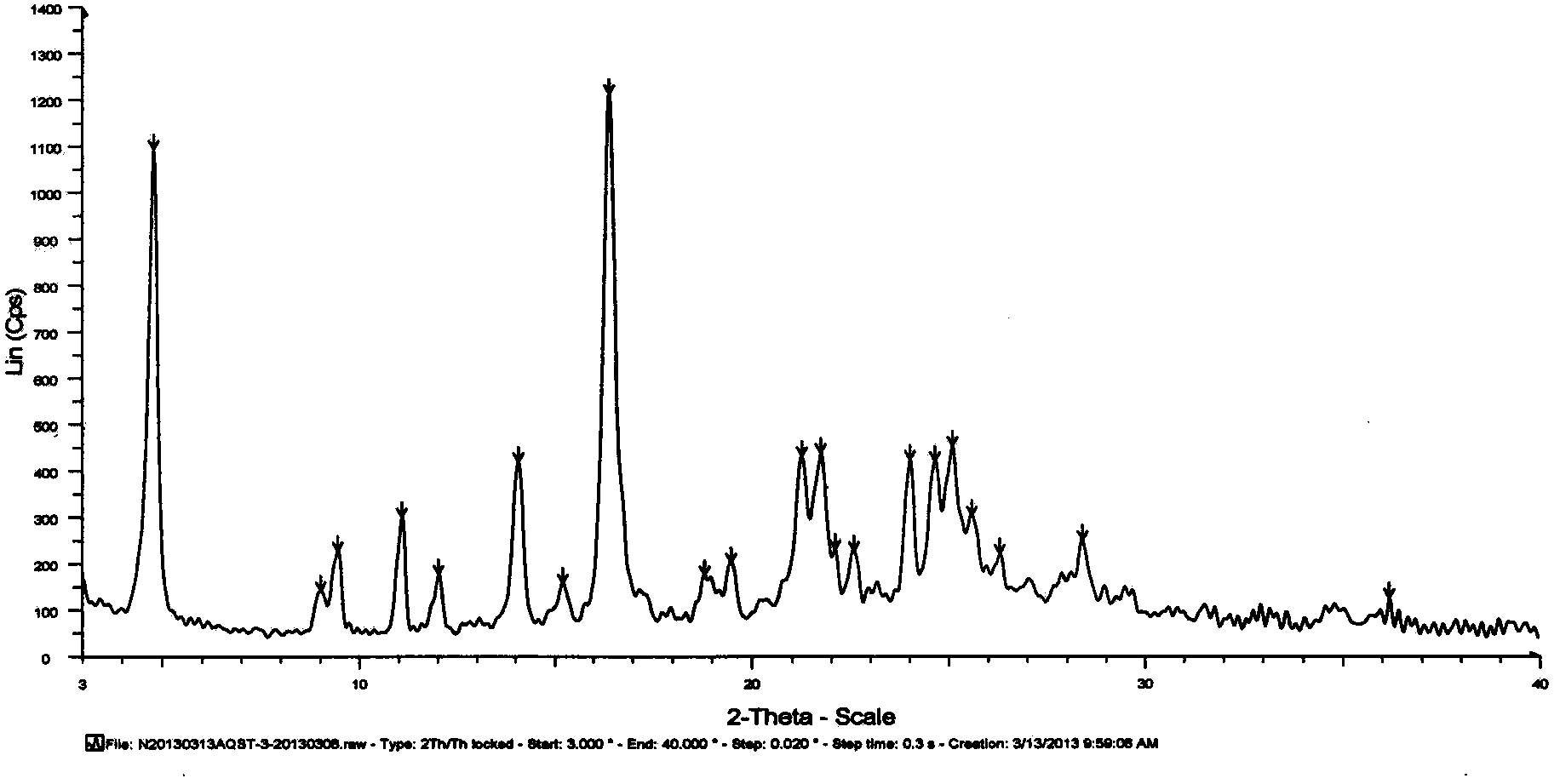
Novel crystal form of azilsartan and preparation method thereof
A technology of crystal form and crystal form, which is applied in the field of type B and its preparation, can solve the problems of solubility and low bioavailability of preparations, and achieve the effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Preparation of Crystal Form B of Azilsartan
[0019] Add 5L of 0.04mol / L sodium hydroxide solution to a 10L three-necked flask, stir mechanically, add 450g of 1-[[2'-(2,5-dihydro-5-oxo-1,2,4oxadiazole-3 -yl)[1,1'-biphenyl]-4-yl]methyl]-2-ethoxy-1H-benzimidazole-7-carboxylic acid. (Temperature control throughout the process to T<20°C), stir to dissolve the solid, add 0.5 L of acetonitrile, stir to obtain a clear and transparent solution, add 1 mol / L hydrochloric acid, adjust the pH to 1-2, precipitate a large amount of solid, stir for 30 min, After suction filtration, the filter cake was rinsed with water until neutral, and the product was air-dried at 45°C to constant weight to obtain 423.7g of the target crystal form B product, with a yield of 94.2%.
Embodiment 2
[0021] Preparation of Crystal Form B of Azilsartan
[0022] Add 3L of 0.1mol / L sodium hydroxide solution to a 10L three-necked flask, stir mechanically, add 450g of 1-[[2'-(2,5-dihydro-5-oxo-1,2,4oxadiazole-3 -yl)[1,1'-biphenyl]-4-yl]methyl]-2-ethoxy-1H-benzimidazole-7-carboxylic acid. Stir to dissolve the solid, add 3L of acetonitrile, stir to obtain a clear and transparent solution, add 1mol / L hydrochloric acid, adjust the pH to 5-6, and precipitate a large amount of solid, stir for 30min, and filter with suction (the temperature is controlled to T<20°C during the whole process) , the filter cake was rinsed with water until neutral, and the product was air-dried at 45° C. to constant weight to obtain 393.5 g of the target product, with a yield of 87.4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com