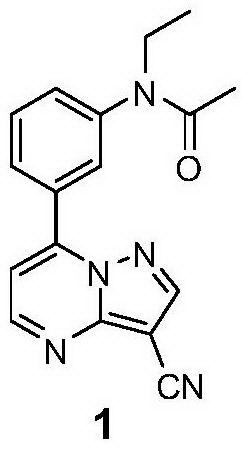
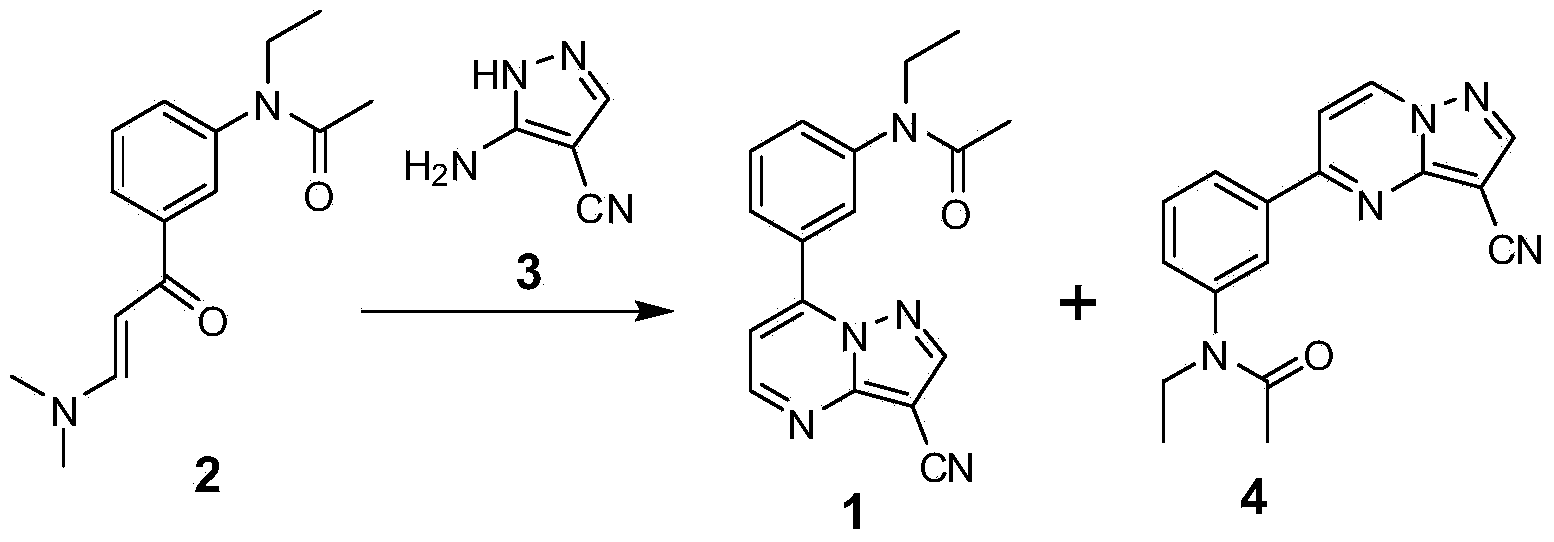
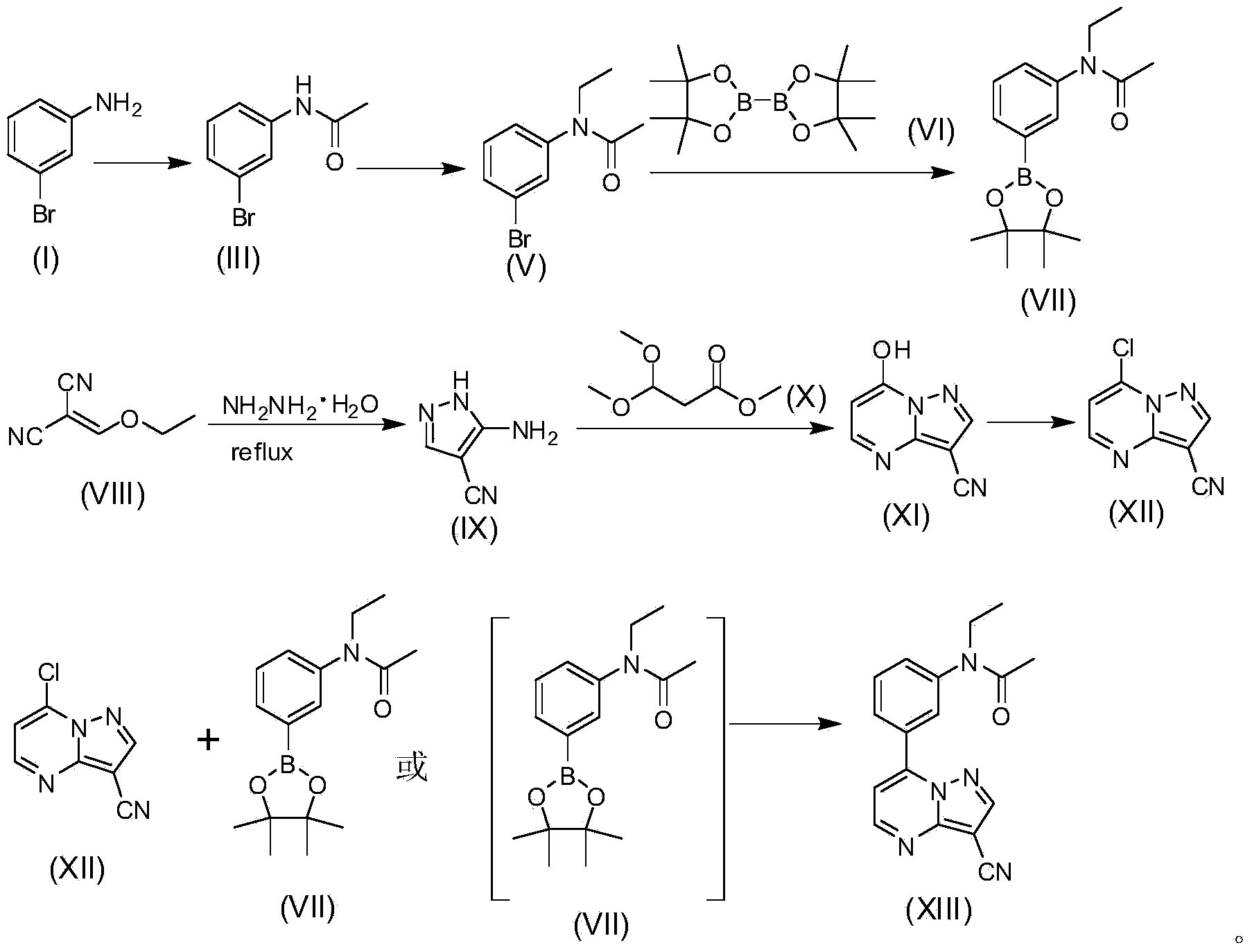
Method for synthesizing zaleplon
A technology for zaleplon and a synthesis method, which is applied in the field of chemical drugs, can solve the problems of poor ring-forming reaction regioselectivity, difficult separation and purification of isomers, unfavorable industrial production and the like, and achieves easy control, easy acquisition and wide application. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Synthesis of compounds of formula (III).
[0023]
[0024] Example 1-1: Add 250 milliliters of dichloromethane, 10.0 grams of formula (I) compound and 11.716 grams of triethylamine in the reaction flask, the resulting solution is cooled to 0°C, and then 5.46 grams of ethyl alcohol are added dropwise to the solution Acid Chlorides (Formula II). The above solution was stirred at room temperature for 3 hours. The reaction solution was washed twice with dilute hydrochloric acid, washed with water, washed with saturated brine, dried and concentrated to obtain 12.0 g of the compound of formula 3 with a yield of 97.3%.
[0025] 1 H NMR (300MHz, CDCl 3 , ppm):7.94(d,J=6Hz,1H),7.54-7.44(m,2H),7.36-7.33(m,4H),7.28-7.24(m,6H),7.11(d,J=6Hz, 2H), 6.92(d, J=6Hz, 2H), 6.76(d, J=6Hz, 6H), 5.09(s, 2H), 4.31(d, J=7.2Hz, 2H), 2.50(t, J= 6Hz, 2H), 1.67-1.55(m, 2H), 1.32-1.24(m, 2H), 0.86(t, J=6Hz, 3H).
Embodiment 2
[0027] Synthesis of compounds of formula (V).
[0028]
example 2-1
[0029] Example 2-1: Add 12 grams of the compound of formula 3, 90 milliliters of N,N-dimethylformamide and 1.22 grams of potassium iodide into a three-necked flask, and then add 4.5 grams of 60% sodium hydrogen. After stirring at room temperature for 30 minutes, 12.18 g of bromoethane (formula IV) were added. The resulting solution was stirred at room temperature for 2 hours, then the reaction solution was poured into ice water, extracted with 200 ml of ethyl acetate, washed 4 times with water, washed once with saturated brine, dried, concentrated, and passed through the column (petroleum ether:ethyl acetate= 5:1) to obtain 11.6 grams of colorless oily liquid, namely the compound of formula (V), with a yield of 85.3%.
[0030] 1 H NMR (300MHz, CDCl 3 ,ppm):9.73(s,1H),7.92(d,J=5.7Hz,1H),7.56-7.40(m,2H),7.35-7.32(m,4H),7.29-7.23(m,6H), 7.10(d, J=6Hz, 2H), 6.92(d, J=6Hz, 6H), 6.83(d, J=6Hz, 2H), 5.45(s, 2H), 2.52(t, J=6Hz, 2H) , 1.72-1.58 (m, 2H), 1.33-1.26 (m, 2H), 0.86 (t, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com