Lithium battery cathode material and preparation method thereof
A positive electrode material and lithium battery technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of low initial efficiency and difficulty in achieving cycle performance, achieve high capacity, improve initial efficiency, and ensure thermal stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The invention provides a method for preparing a positive electrode material of a lithium battery, comprising the following steps:
[0022] S1, first pump the solution A and the alkali solution into the reaction vessel at the same speed, and carry out the co-precipitation reaction;
[0023] S2. After step S1 is carried out for 5-25 hours, pump solution B into solution A at a constant speed, and pump the whole mixed solution and alkali solution into the reaction vessel at a constant speed while pumping solution B, and continue the co-precipitation reaction;
[0024] S3. After step S2 is completed, the solution C and the alkali solution are pumped into the reaction container at a constant speed, and the co-precipitation reaction is continued, and the precursor is obtained by drying after the reaction is terminated;
[0025] S4. Mix the precursor obtained in step S3 with lithium salt, and obtain the lithium battery positive electrode material after sintering;
[0026] The ...
Embodiment 1
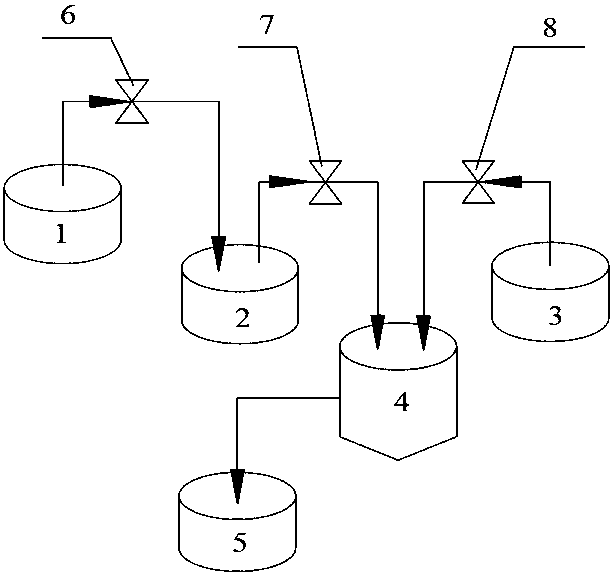
[0055] (1) if figure 1 As shown, the solution A1 of 2mol / L is charged in the second container 2, and the solution A1 is an aqueous solution of nickel sulfate, manganese sulfate and cobalt sulfate, and the molar ratio Ni:Co:Mn=8:1:1; A container 1 is filled with 2mol / L solution B1, which is an aqueous solution of nickel sulfate, manganese sulfate and cobalt sulfate, and the molar ratio Ni:Co:Mn=4:3:3. Load alkaline solution into the third container 3, the alkaline solution is a mixed system of sodium hydroxide and ammoniacal liquor, the concentration of sodium hydroxide in the alkaline solution is 2mol / L, and the concentration of ammoniacal liquor is 1mol / L;
[0056] (2) Turn off the first pump 6, turn on the second pump 7 and the third pump 8, and pump the solution A1 in the second container 2 and the alkali solution in the third container 3 into the reaction container 4 at the same speed The reaction is carried out in the middle, and the reaction time is 10h; At this moment,...
Embodiment 2
[0061] The same steps as in Example 1 were used to prepare the lithium battery positive electrode material S20 of this example, except that:
[0062] In step (1), solution A2 is an aqueous solution of nickel nitrate, manganese nitrate and cobalt nitrate, the molar ratio Ni:Co:Mn=5:2.5:2.5, the concentration of solution A2 is 3mol / L; solution B2 is nickel nitrate, nitric acid The aqueous solution of manganese and cobalt nitrate, the molar ratio Ni:Co:Mn=1:4.5:4.5, the concentration of solution B2 is 0.6mol / L;
[0063] In step (2), the reaction time is 15 hours; at this time, the remaining solution A2 in the second container 2 is equal to the volume of the solution B2 contained in the first container 1;
[0064] In step (3), the reaction time is 2h;
[0065] In step (4), the solution C2 is a 3mol / L cobalt nitrate solution; the reaction time is 2h.
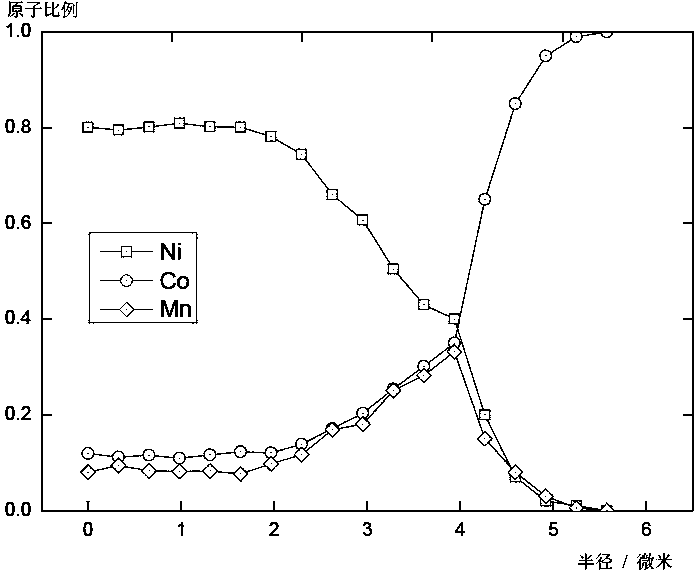
[0066] Through the above steps, the co-precipitation precursor S2 (its chemical formula is Ni 0.48 co 0.27 mn 0.25 (OH) 2 ) and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com