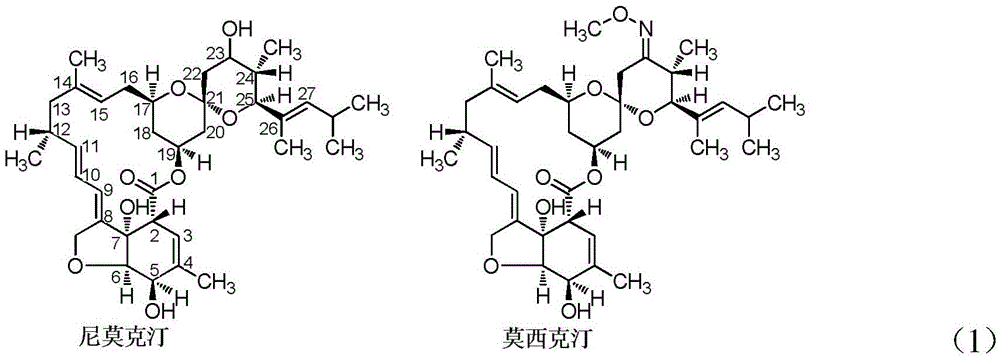
A kind of membrane separation preparation method of high-purity moxictine
A high-purity moxictine technology, which is applied in the field of membrane separation and preparation of high-purity moxictine, can solve the problems of high investment cost and production cost, complex process, high energy consumption, etc., and reduce production cost and investment cost , Improve product purity, low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The method steps for preparing high-purity moxectine in this embodiment are as follows:
[0029] (1) The fermentation broth produced by Streptomyces fermentation is separated by solid-liquid, and the pre-purified solution of nemoctine is obtained after leaching, with a purity of 15.6%, containing 80g of solids with a concentration of 1.5%, pumped into polyethersulfone hollow fiber microfiltration Membrane filtration, control pump pressure 0.1-0.7MP. The membrane filtrate is received to obtain a filtrate with a purity of 20%.
[0030] The above-mentioned filtrate is pumped into a 3000 molecular weight cut-off ultrafiltration membrane to filter, the pump pressure is controlled to be 0.5-0.9MP, and the filtrate is received to obtain a filtrate with a purity of 52.5%.
[0031] (2) After step (1), the ultrafiltration nimoxetine solution is pumped into a roll nanofiltration membrane with a molecular weight cut-off of 200 for concentration to obtain a nimoxetine concentrate.
[0032]...
Embodiment 2
[0038] The method steps for preparing high-purity moxectine in this embodiment are as follows:
[0039] (1) After solid-liquid separation of the fermentation broth produced by Streptomyces, the pre-purified nemoctine liquid with a purity of 18.2% and a solid content of 1.8% in 90g is pumped into the polyethersulfone hollow fiber microfiltration. Membrane filtration, control pump pressure 0.1-0.7MP. The membrane filtrate is received to obtain a filtrate with a purity of 20%.
[0040] The above-mentioned filtrate is pumped into a 2000 molecular weight cut-off ultrafiltration membrane filtration, the pump pressure is controlled to 0.7-1.2MP, and the filtrate is received to obtain a filtrate with a purity of 52%.
[0041] (2) After step (1), the ultrafiltration nimoxetine solution is pumped into a roll nanofiltration membrane with a molecular weight cut-off of 200 for concentration to obtain a nimoxetine concentrate.
[0042] (3) Add the nimoxetine concentrate obtained in step (2) to dic...
Embodiment 3
[0048] The method steps for preparing high-purity moxectine in this embodiment are as follows:
[0049] (1) The fermentation broth produced by the fermentation of Streptomyces is separated from the solid and liquid, and the pre-purified liquid of nemoctine obtained after leaching, with a purity of 13.2%, containing 100g of solids with a concentration of 1.4.8%, is pumped into the polyethersulfone control Filtration by fiber microfiltration membrane, control pump pressure 0.1-0.7MP. The membrane filtrate was received to obtain a filtrate with a purity of 18.6%.
[0050] The above-mentioned filtrate is pumped into a 1500 molecular weight cut-off ultrafiltration membrane filtration, the pump pressure is controlled to be 0.7-1.2MP, and the filtrate is received to obtain a filtrate with a purity of 51.4%.
[0051] (2) After step (1), the ultrafiltration nimoxetine solution is pumped into a roll nanofiltration membrane with a molecular weight cut-off of 200 for concentration to obtain a n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com