A method for detecting related substances of ambrisentan
A technology related to substances and detection methods, applied in the field of analytical chemistry, can solve problems such as ghost peaks, inaccurate detection results, incomplete separation, etc., and achieve the effects of high precision, high peak symmetry, and high system adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: detection method of the present invention
[0052] Instrument: Shimadzu high performance liquid chromatography
[0053] Chromatographic column: WatersAtlantisT3 (4.6×250mm, 5μm)
[0054] Mobile phase A: 0.02% trifluoroacetic acid in water
[0055] Mobile phase B: 0.01% trifluoroacetic acid in acetonitrile
[0056] Detection wavelength: 220nm
[0057] Flow rate: 1.0ml / min
[0058] Injection volume: 20μl
[0059] Column temperature: 30°C
[0060] Autosampler temperature control: 5°C
[0061] The elution program is shown in Table 1:
[0062] Table 1 Elution program
[0063] time (min)
[0064] The test solution: take 12.5mg of ambrisentan sample, put it in a 50ml measuring bottle, add 12.5ml of 0.01% trifluoroacetic acid acetonitrile solution to dissolve, add 0.02% trifluoroacetic acid aqueous solution to dilute to the mark, shake well, and use it as the test sample solution.
[0065] Reference substance solution: Take 1.0ml of the test so...
Embodiment 2
[0072] Embodiment 2: detection method system adaptability detection of the present invention
[0073] Dilute solution: 0.01% trifluoroacetic acid in acetonitrile solution - 0.02% trifluoroacetic acid aqueous solution (25:75) (V / V)
[0074] Blank solution: dilute solution
[0075] Impurity stock solution: Take 12.5mg each of impurities SRS1, SRS2, SRS3, SRS4, and dp1, accurately weigh them in a 100ml measuring bottle, dissolve them with diluent solution and dilute to the mark, and shake well.
[0076] Resolution solution: Take about 12.5mg of ambrisentan, put it in a 50ml measuring bottle, add 12.5ml of 0.01% trifluoroacetic acid acetonitrile solution to dissolve, add 1.0ml of impurity stock solution, dilute to the mark with 0.02% trifluoroacetic acid aqueous solution, Shake well.
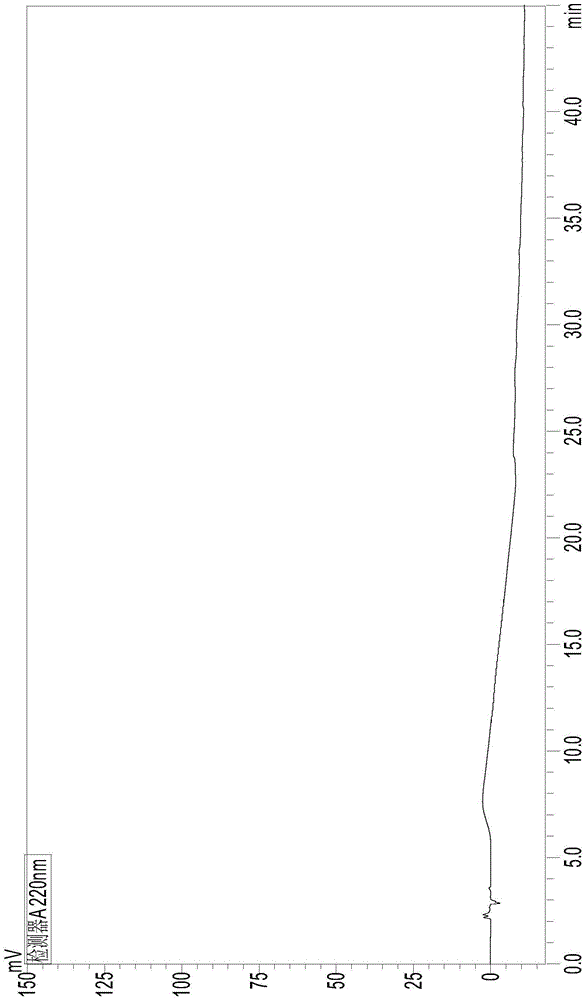
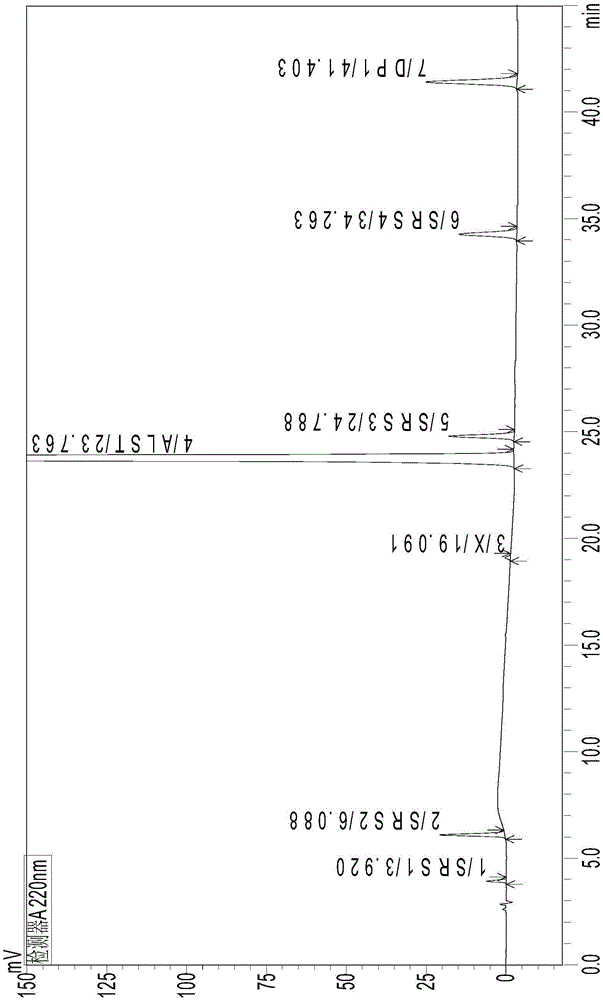
[0077] According to the chromatographic conditions of Example 1, sample blank solution and resolution solution were respectively injected, and the chromatogram was recorded, see figure 1 and fi...
Embodiment 3
[0082] Embodiment 3: Specific detection of the detection method of the present invention
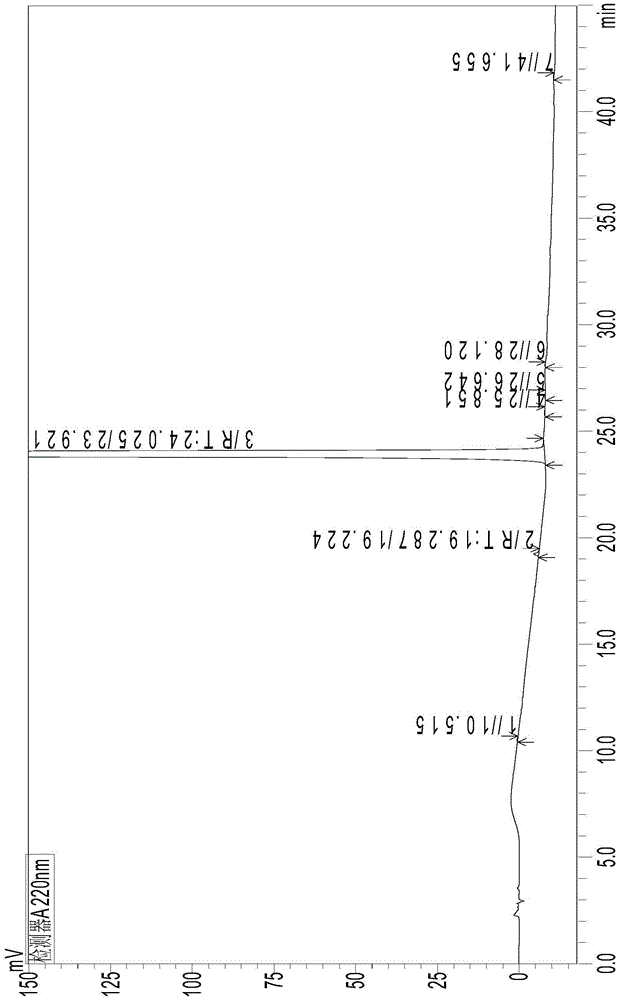
[0083] The strong degradation test is to accelerate the destruction of ambrisentan under strong conditions, such as strong acid, strong alkali, strong oxidation, high temperature, and strong light. The separation status of the analysis method is compared with the amount of impurities generated and the reduction of the main component to evaluate the effectiveness and applicability of the analytical method. At the same time, the DAD detector is used to check the peak purity: in the spectrum obtained from the degradation test, when the purity factor of the main component is greater than the threshold, it can be judged that the chromatographic peak does not contain other impurity peaks, and the chromatographic peak purity meets the requirements. The specific test results are shown in the table 4. The corresponding chromatograms are shown in Figure 3-8 .
[0084] Table 4 specificity test r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| percent by volume | aaaaa | aaaaa |
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com