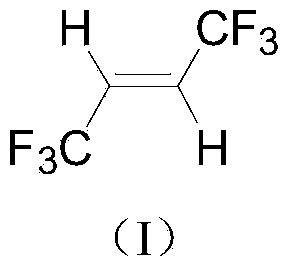
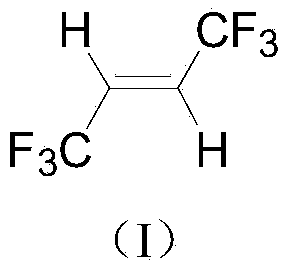
Synthetic method of trans-1, 1, 1, 4, 4, 4-hexafluoro-2-butene
A synthesis method and technology of butene, applied in the direction of halogen substitution preparation, dehydrohalogenation preparation, organic chemistry, etc., can solve the problems of high price, large pollution, low selectivity, etc., and achieve the effect of high price and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (a) Add 1.4 grams of copper chloride and 3.2 grams of 2,2-bipyridine to a 500 mL titanium alloy belt stirred autoclave, dissolve them in 20 mL of methanol, replace the air in the kettle with nitrogen, and inject 312 grams of R123 and 63 g CH 2 =CHCl, reaction temperature 120°C, reaction pressure 1.2MPa, reaction time 10 hours, remove R123 and methanol from the crude product after reaction by distillation at atmospheric pressure, recycle R123 and methanol, continue vacuum distillation to collect CF 3 CHClCH 2 CHCl 2 , conversion rate 91.3%, selectivity 87.6%.
[0032] (b) Add 150 g of CF to a 500 mL quartz photochemical reactor 3 CHClCH 2 CHCl 2 Dissolve in 300mL carbon tetrachloride solution, slowly pass in about 140 grams of chlorine gas under stirring, react at 0°C for 24 hours, and continue to collect the product CF after vacuum distillation to separate carbon tetrachloride 3 CHClCH 2 CCl 3 , conversion rate 90.5%, selectivity 78.7%.
[0033] (c) In a nickel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com