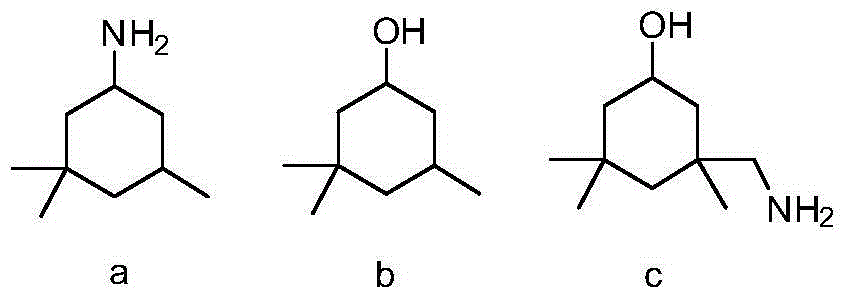
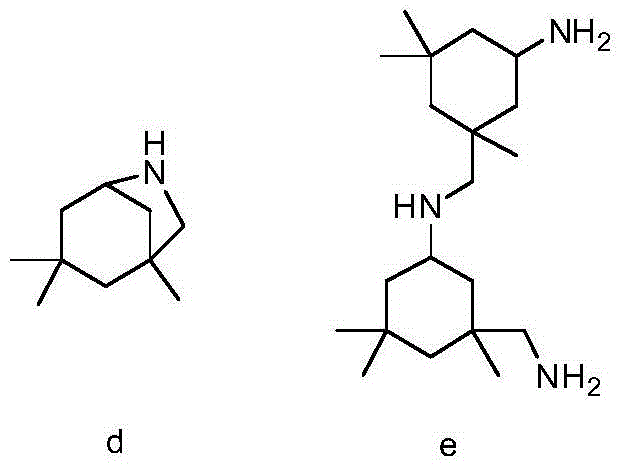
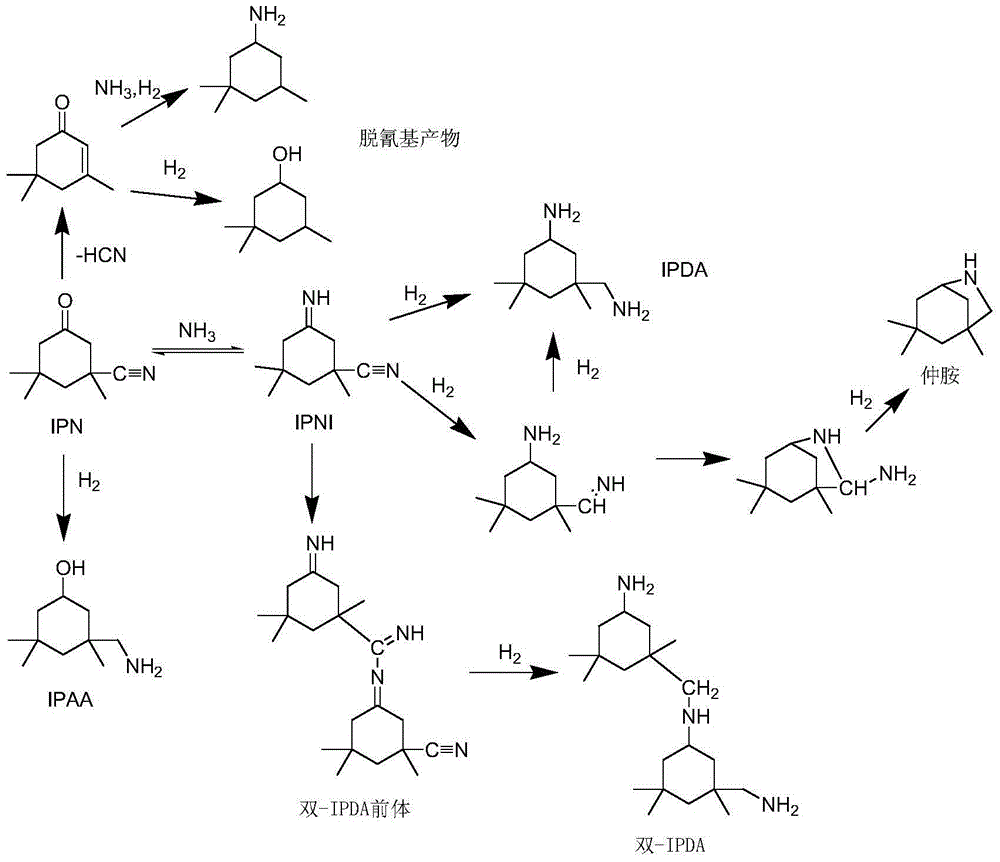
Preparation method of 3-aminomethyl-3,5,5-trimethyl cyclohexylamine
A technology of trimethylcyclohexylamine and trimethylcyclohexanone, which is applied in the field of preparation of aliphatic diamines, can solve the problems of limited IPDA yield, low space velocity in the imidization step, and formation of aminoalcohol bis-IPDA Large volume and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] a) The acid value of IPN is controlled at 0.30mgKOH / g, and is fed into the primary imidization reactor 1 together with liquid ammonia, the IPN feed rate is 700g / h, the feed rate of liquid ammonia is 2850g / h, and the hydrogen flow rate 9500 standard L / h, the temperature is controlled at 40°C, and the reaction pressure is 20MPa to obtain a partially imidized reaction solution;
[0068] b) Add NaOH solid with an initial IPN molar mass of 3‰ to the partially imidized reaction solution before entering the secondary imidization reactor 2, and then enter the secondary imidization reactor 2, with the temperature controlled at 20°C , the reaction pressure is 20MPa;
[0069]c) The product obtained in step b) is fed into the hydrogenation reactor 3, the inlet temperature of the hydrogenation reactor 3 is controlled at 80° C., the outlet temperature is controlled at 100° C., and the reaction pressure is 20 MPa. The space velocity above the catalyst in each reactor is shown in Tabl...
Embodiment 2
[0076] a) The acid value of IPN is controlled at 0.15mgKOH / g, and is fed into the primary imidization reactor 1 together with liquid ammonia, the feed rate of IPN is 800g / h, the feed rate of liquid ammonia is 1645g / h, and the temperature is controlled At 50°C, with a reaction pressure of 15MPa, a partially imidized reaction solution was obtained;
[0077] b) Add LiOH solid with an initial IPN molar mass of 1.5‰ to the partially imidized reaction solution before entering the secondary imidization reactor 2, and then enter the secondary imidization reactor 2, with the temperature controlled at 30°C , the reaction pressure is 15MPa;
[0078] c) The product obtained in step b) was fed into the hydrogenation reactor 3, and hydrogen gas was introduced at a flow rate of 2170 standard L / h. The inlet temperature of the hydrogenation reactor 3 was controlled at 80° C., the outlet temperature was controlled at 100° C., and the reaction pressure was 15 MPa. The space velocity above the c...
Embodiment 3
[0085] a) The acid value of IPN is controlled at 0.05mgKOH / g, and is fed into the primary imidization reactor 1 together with liquid ammonia, the IPN feed rate is 1000g / h, the feed rate of liquid ammonia is 5150g / h, and the hydrogen flow rate 10,000 standard L / h, the temperature is controlled at 60°C, and the reaction pressure is 20MPa to obtain a partially imidized reaction solution;
[0086] b) Add NaOH solid with an initial IPN molar mass of 0.5‰ to the partially imidized reaction solution before entering the secondary imidization reactor 2, and then enter the secondary imidization reactor 2, with the temperature controlled at 40°C , the reaction pressure is 20MPa;
[0087] c) The product obtained in step b) is fed into the hydrogenation reactor 3, the inlet temperature of the hydrogenation reactor 3 is controlled at 90° C., the outlet temperature is controlled at 120° C., and the reaction pressure is 20 MPa. The space velocity above the catalyst in each reactor is shown i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com