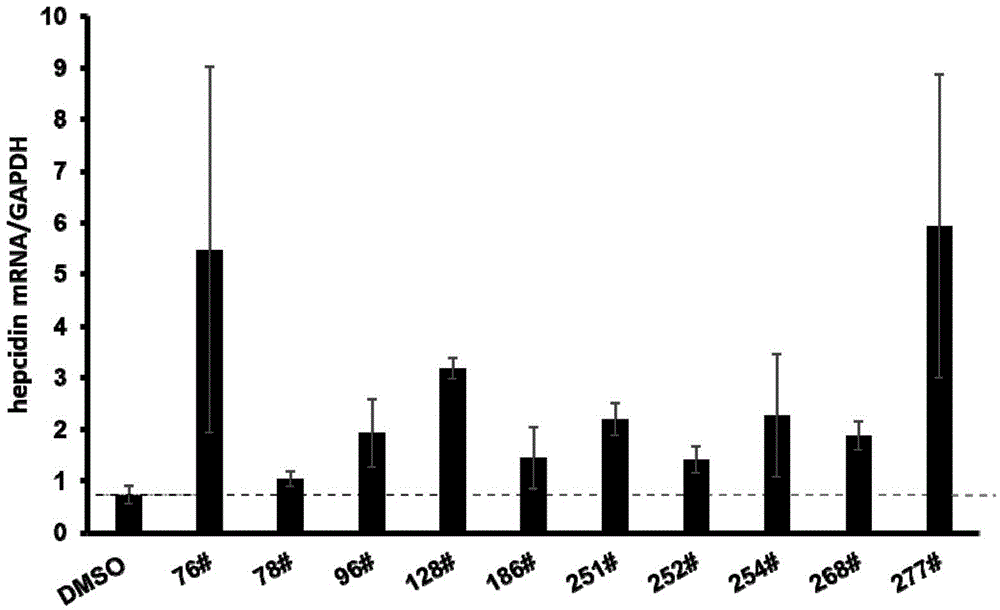
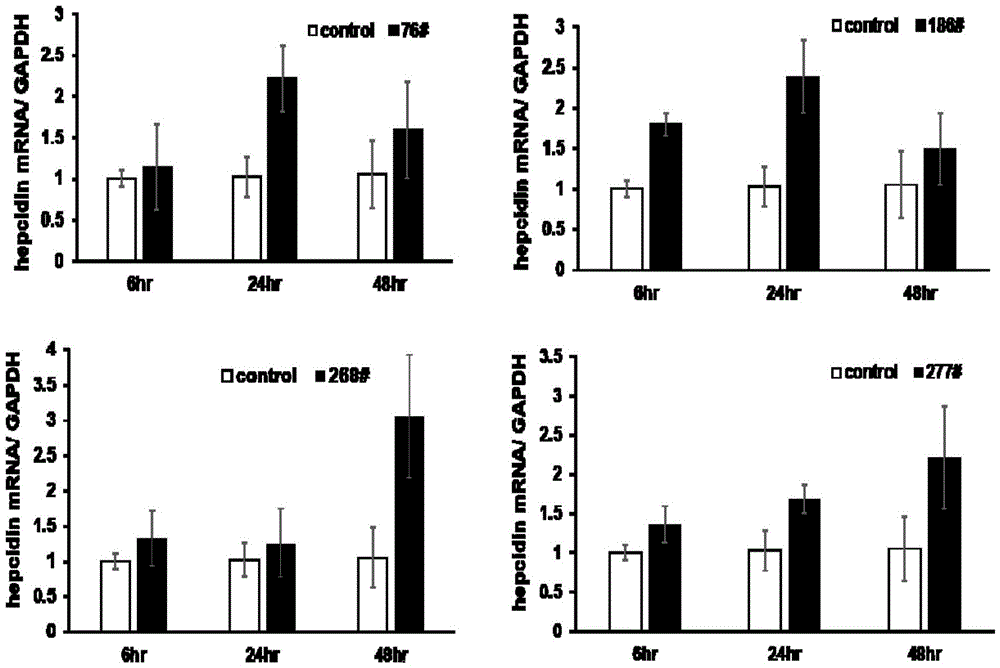
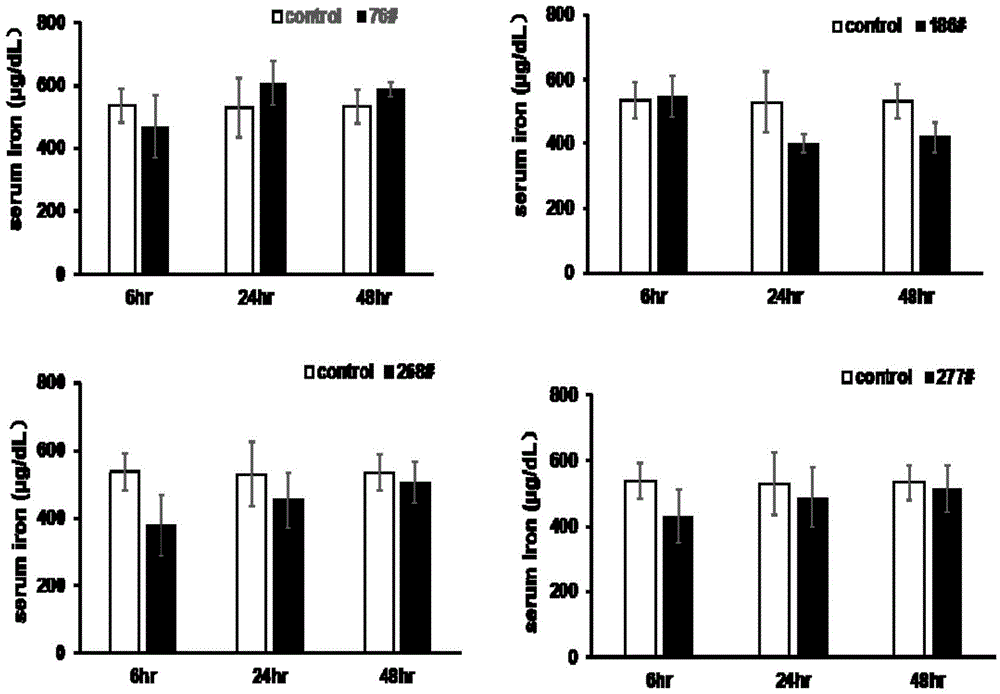
Thiazolidone compound and application of thiazolidone compound in preparation of medicine used for treating iron disorder-related diseases
A technology of thiazolidinones and compounds, applied in the field of thiazolidinone derivatives and their applications, can solve the problems of inhibiting bone marrow hyperplasia, easily causing infection, liver damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of 2-(2-chlorophenylimino)-5-(4-hydroxy-3-methoxybenzylidene)-1,3-thiazol-4-one
[0044]
[0045] by HCl:H 2 The ratio of O (1:4) is dubbed hydrochloric acid solution. Weigh 11.4g (150mmol) of ammonium thiocyanate and dissolve it in 50mL of hydrochloric acid solution, add 10.5mL (100mmol) of 3-toluidine, heat to 85°C under stirring conditions, the mixture becomes clear, after 12 hours of reaction, TLC monitors the reaction, After the reaction, the mixture was cooled to room temperature, and a viscous oily liquid appeared, which was extracted with ethyl acetate, and the extract was washed with 10% hydrochloric acid solution, saturated sodium chloride solution, and water successively, and the solvent was evaporated off the extract under reduced pressure to obtain 2-Tolylthiourea.
[0046] Add 996mg (6.0mmol) of 2-tolylthiourea and 2479mg (30mmol) of anhydrous sodium acetate into 20mL of ethanol, add 1.28mL (12mmol) of ethyl chloroacetate under stirring co...
Embodiment 2
[0050] 76#.2-(2-chlorophenyl)-5-(9H-fluorenylbenzylidene)-1,3-thiazol-4-one
[0051] The preparation method is similar to the preparation of the compound of Example 1.
[0052] The product is a yellow powder with a yield of 75.5%; 1 HNMR (400MHz, CDCl 3 )δ(ppm)=8.0(s,1H),7.85(m,2H),7.72(s,1H),7.45(m,9H),4.12(d,2H).C 23 h 15 ClN 2 OS, ESI-MS: m / z 403.7 (M+1) + .
Embodiment 3
[0054] 78#.2-(2-Chlorophenyl)-5-(4-methylthiobenzylidene)-1,3-thiazol-4-one
[0055] The preparation method is similar to the preparation of the compound of Example 1.
[0056] The product is an orange powder with a yield of 92.6%; 1 HNMR (400MHz, CDCl 3 )δ(ppm)=8.2(s,1H),7.72(s,1H),7.49(d,1H),7.35(m,7H),2.53(s,3H).C 17 h 13 ClN 2 OS 2 ,ESI-MS:m / z 360.0(M+1) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com