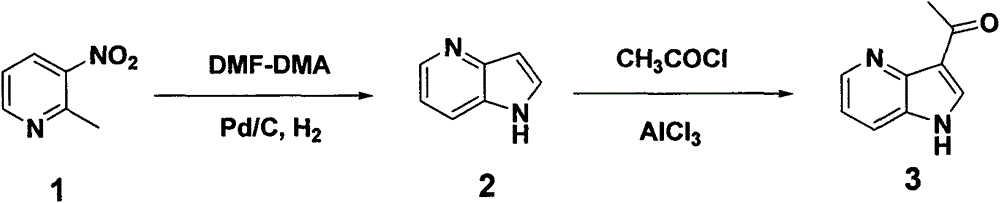
Practical synthesis method of 3-acetyl-4-azaindole
A technique for the synthesis of azaindole, which is applied in the fields of medicine and chemical industry, can solve the problems of unavailable products, unsuitable for scale-up and industrial production, and low reaction yield, and achieve mild conditions, shorten the production process, and improve the reaction rate. The effect of temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
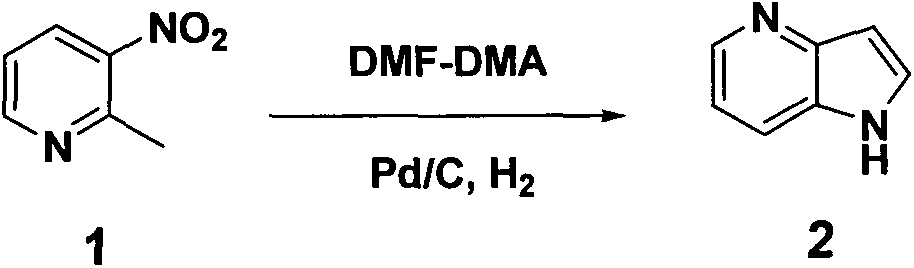
[0015] Embodiment 1: synthetic 4-azaindole (compound 2)
[0016]
[0017] 2-Methyl-3-nitropyridine (23.4 g, 0.17 mol), DMF-DMA (60 g, 0.51 mol) and DMF (100 mL) were heated to 90° C. and stirred for 8 hours. Cool to room temperature, pour the reaction solution into 300 ml of cold water, filter with suction to obtain a red solid, dissolve the solid in ethanol (500 ml), add 10% Pd / C (5 g), and stir at room temperature for 10 hours under a hydrogen atmosphere , suction filtration (spread a layer of diatomaceous earth), wash with ethanol, and concentrate to obtain 18.1 g of brown solid 4-azaindole, yield: 90.5%.
[0018] EI-MS MS (m / z): 119.1 (M + )
[0019] 1 H-NMR (DMSO-d6, 400MHz): δ8.29~8.27(m, 1H), 7.76(d, 1H), 7.61(d, 1H), 7.08~7.04(m, 1H), 6.54~6.52(m , 1H).
Embodiment 2
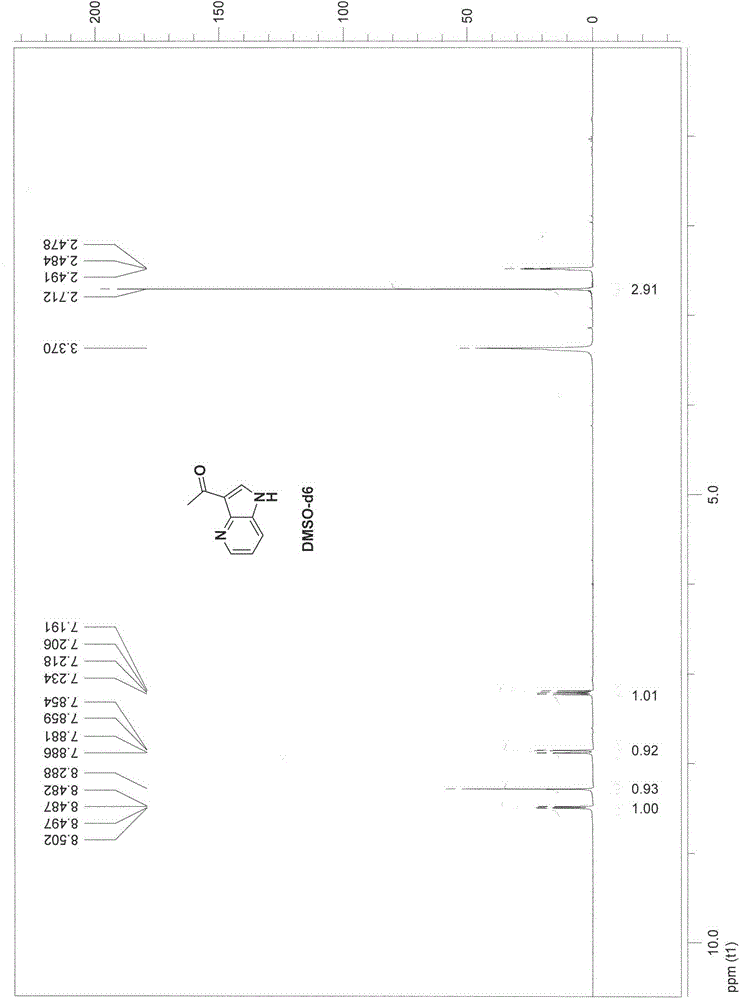
[0020] Embodiment 2: Synthesis of 3-acetyl-4-azaindole (compound 3)
[0021]
[0022] Add 4-azaindole (21g, 0.18mol) to anhydrous aluminum chloride (106.8g, 0.80mol) and suspend in dichloroethane (800ml), heat to 50°C and stir for 1 hour, then cool to room temperature , dropwise added acetyl chloride (18.8g, 0.24 moles), heated to 50°C and stirred for 2 hours after the addition, cooled, slowly added dropwise methanol (300 ml) to quench the reaction, concentrated to dryness, added water (100 ml), Add sodium carbonate solution dropwise until a large amount of solids precipitate, filter with suction, wash the solid with ethyl acetate (400 ml), discard the solid, wash the ethyl acetate layer with saturated brine (50 ml), dry over anhydrous sodium sulfate, and filter with suction , concentrated to obtain a crude product, added n-hexane (60 ml), stirred, suction filtered, and dried to obtain 20.2 g of off-white solid 3-acetyl-4-azaindole, yield: 70.1%.
[0023] EI-MS MS (m / z): 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com