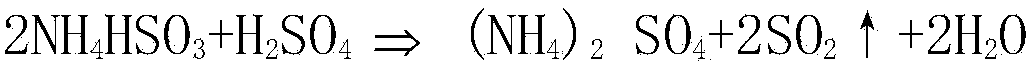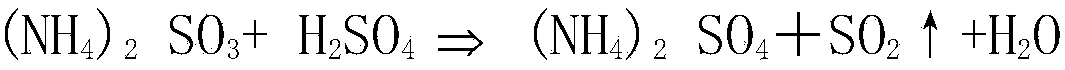Method for producing liquid sulfur dioxide by using sulfur-containing flue gas
A liquid sulfur dioxide and flue gas technology, applied in sulfur dioxide/sulfurous acid, chemical instruments and methods, specific gas purification/separation, etc., can solve problems such as single product, reduce production costs, improve economic benefits, and do not produce secondary The effect of pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
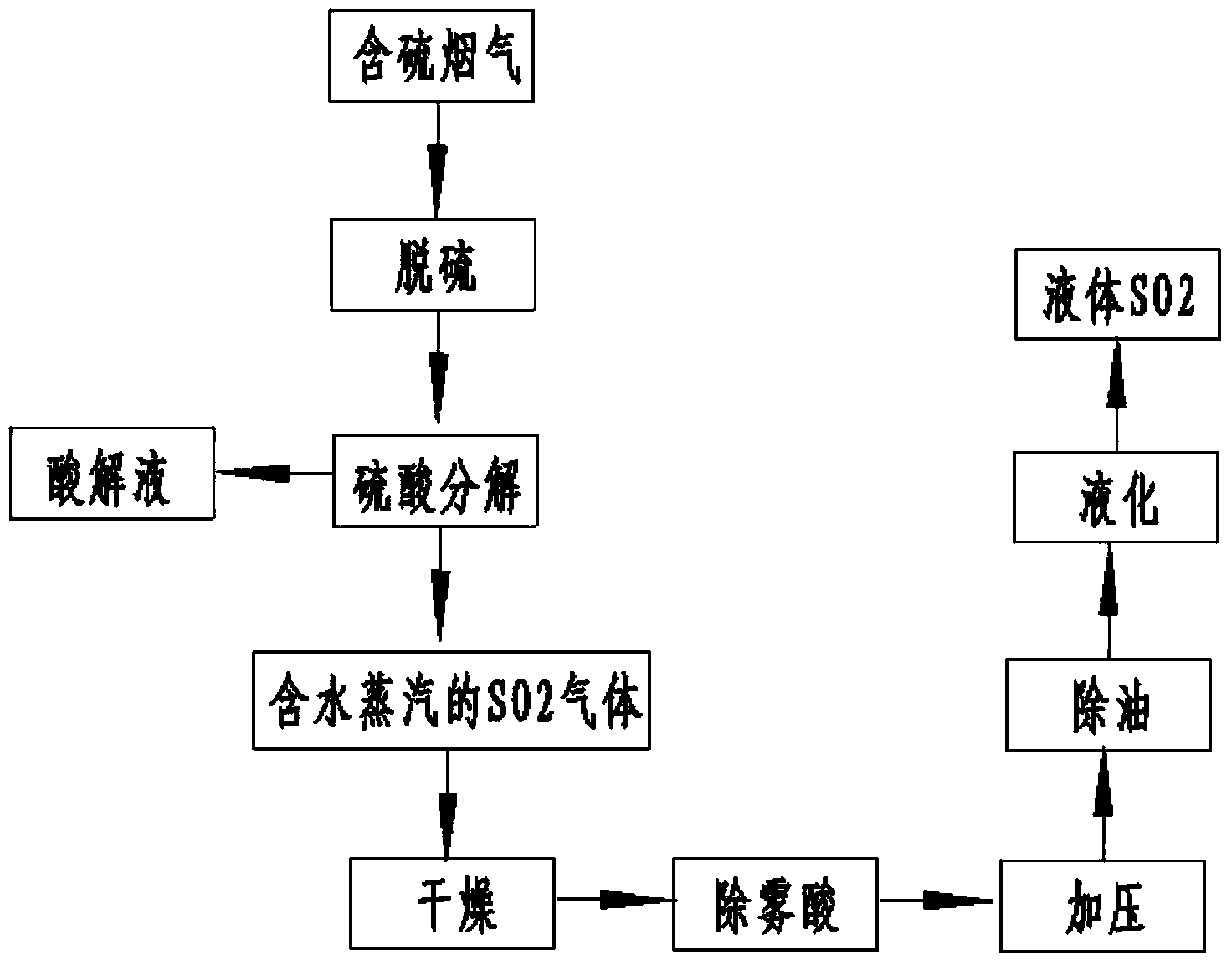
[0026] a. The sulfur-containing flue gas (concentration of sulfur dioxide is 13210mg / Nm 3 ) is absorbed through ammonium sulfite solution (concentration is 254g / L) to obtain sulfur-containing absorption liquid; the concentration of sulfur dioxide in the absorbed sulfur-containing flue gas is 30mg / Nm 3 .
[0027] b. Decompose the sulfur-containing absorption solution by adding sulfuric acid with a concentration of 95% by volume to obtain acidolysis solution and SO containing water vapor 2 gas;
[0028] c, the SO containing water vapor 2 The gas is spray dried with sulfuric acid to SO 2 Gas water content≤0.2g / Nm 3 ; The amount of sulfuric acid spraying is 140m 3 / h. Removal of dry SO using nanofiber mist eliminators in drying 2 Acid mist brought by the gas process.
[0029] d. The dried SO 2 The gas is pressurized under the pressure condition of 0.25-0.7MPa, deoiled and liquefied to obtain liquid sulfur dioxide. The output is 116.14kg / h.
Embodiment 2
[0031] a. The sulfur-containing flue gas (concentration of sulfur dioxide is 14035mg / Nm 3 ) is absorbed through ammonium sulfite solution (concentration is 245g / L) to obtain sulfur-containing absorption liquid; the concentration of sulfur dioxide in the sulfur-containing flue gas after absorption is 35mg / Nm 3 .
[0032] b. Decompose the sulfur-containing absorption solution by adding sulfuric acid with a concentration of 90% by volume to obtain acidolysis solution and SO containing water vapor 2 gas;
[0033] c, the SO containing water vapor 2 The gas is spray dried with sulfuric acid to SO 2 Gas water content≤0.2g / Nm 3 ; The amount of sulfuric acid spraying is 160m 3 / h. Removal of dry SO using nanofiber mist eliminators in drying 2 Acid mist brought by the gas process.
[0034] d. The dried SO 2 The gas is pressurized under the pressure condition of 0.25-0.7MPa, deoiled and liquefied to obtain liquid sulfur dioxide. The output is 117.33kg / h.
Embodiment 3
[0036] a. The sulfur-containing flue gas (concentration of sulfur dioxide is 14655mg / Nm 3 ) is absorbed through ammonium sulfite solution (concentration is 251g / L) to obtain sulfur-containing absorption liquid; the concentration of sulfur dioxide in the sulfur-containing flue gas after absorption is 40mg / Nm 3 .
[0037] b. Decompose the sulfur-containing absorption solution by adding sulfuric acid with a concentration of 98.5% by volume to obtain acidolysis solution and SO containing water vapor 2 gas;
[0038] c, the SO containing water vapor 2 The gas is spray dried with sulfuric acid to SO 2 Gas water content≤0.2g / Nm 3 ; The amount of sulfuric acid spraying is 120m 3 / h. Removal of dry SO using nanofiber mist eliminators in drying 2 Acid mist brought by the gas process.
[0039] d. The dried SO 2 The gas is pressurized under the pressure condition of 0.25-0.7MPa, deoiled and liquefied to obtain liquid sulfur dioxide. The output is 115.67kg / h.
[0040] In the abov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com