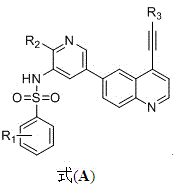
Benzene sulfonamide derivatives, preparation method, and treatment application
A technology of benzenesulfonamide and derivatives, which is applied in the field of medicine and can solve problems such as high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] N-(5-(4-(3-(dimethylamino)prop-1-yn-1-yl)quinolin-6-yl)-2-methoxypyridin-3-yl)-2,4 - Difluorobenzenesulfonamide (III, compound number in the table 1 ) preparation
[0063]
[0064] a) Preparation of N-(5-(4-chloroquinolin-6-yl)-2-methoxypyridin-3-yl)-2,4-difluorobenzenesulfonamide (II)
[0065] Dissolve 6-bromo-4-chloroquinoline (2 g, 8.25 mmol) in dioxane (30 mL), add pinacol diborate (2.30 g, 9.07 mmol) and potassium acetate (1.62 g, 16.50 mmol ) and the palladium catalyst PdCl 2 (dppf)CH 2 Cl 2 (0.21g, 0.25mmol), heated to reflux at 100°C for 3h under the protection of nitrogen, the raw material point was detected to disappear, and the reaction was stopped. Add N-(5-bromo-2-methoxypyridin-3yl)-2,4-difluorobenzenesulfonamide (I) (3.4 g, 9.08 mmol) and another part of palladium catalyst PdCl to the mixed system 2 (dppf)CH 2 Cl 2 (0.21 g, 0.25 mmol) and 2M K 2 CO 3 solution (10 mL), heated to reflux at 110° C. for 16 h under nitrogen protection to stop the ...
Embodiment 2-11
[0069] surface 1 Compound 2-11 Can be by corresponding raw material, with reference to preparation embodiment 1 Compounds were prepared by general methods.
Embodiment 12
[0070] Example 12 : The enzyme inhibitory activity assay of this series of compounds:
[0071] The test results of the enzyme inhibitory activity of the above-mentioned compounds are shown in the table 3 , a total of three methods were used in the test:
[0072] Using the method of Lance Ultra Assay to test the IC of mTOR 50 value.
[0073] Using the method of ADP-Glo Luminescent Kinase Assay to test the IC of PI3Kβ and PI3Kγ 50 value.
[0074] Use the method of Kinase-Glo Luminescent Kinase Assay to test the IC of PI3Kα and PI3Kδ 50 value.
[0075] Table 3 IC of some compounds on PI3Kα 50 (nM)
[0076]
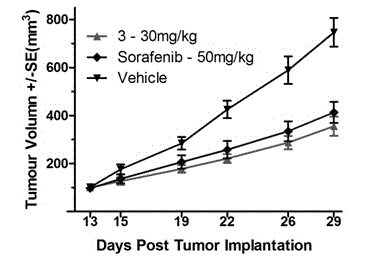
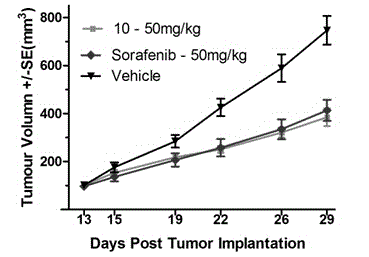
[0077] Table 3 It can be seen that all the synthesized compounds have good inhibitory activity on PI3Kα, the best of which is the compound 3 , is 2.0 nM. On this basis, two compounds with better activity were preferred, and their inhibitory activities on mTOR and different subtypes of PI3K were tested, and it was shown that they had inhibitory activit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com