Preparation method of atropine sulphate
A technology of atropine sulfate and tropin ester, applied in the direction of organic chemistry, etc., can solve the problems of unfavorable increase in product yield, cumbersome operation, high cost, etc., achieve simplification of salt formation and post-treatment steps, simple process operation, and reduce production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
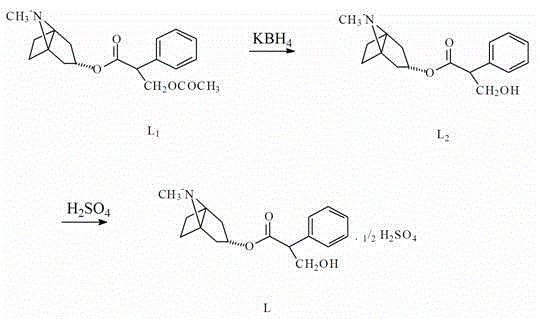
[0024] Add 50 ml of methanol, 200 ml of chloroform, and 20 g (0.06 mol) of α-formylphenylacetic acid tropinate into the reaction flask and stir evenly. Add 5 g of potassium borohydride (0.09 mol), stirred and hydrolyzed for 4 hours. Add 200 ml of water to the above mixture, separate the chloroform organic layer, extract the water layer once with chloroform and merge into the original chloroform layer, after the chloroform layer is distilled to recover chloroform, freeze and crystallize for 2 hours, filter to obtain 16.9 g (0.058mol ).
[0025] Put 16.9 g (0.058 mol) of the crude product of atropine, 30 ml ethanol, and 100 ml acetone into the reaction bottle, stir, add sulfuric acid dropwise in an ice-water bath at 0-10°C to adjust the pH of the solution to 5-6, refrigerate overnight for crystallization, filter, 16.1 g (0.049 mol) of atropine sulfate were obtained.
Embodiment 2
[0027] Add 30 ml of methanol, 200 ml of chloroform, and 20 g (0.06 mol) of α-formylphenylacetic acid tropinate into the reaction flask and stir evenly. Add 6.5 g of potassium borohydride (0.12 mol), stirred and hydrolyzed for 4 hours. Add 200 ml of water to the above mixture, separate the chloroform layer, extract the water layer once with chloroform and merge into the original chloroform layer, recover the chloroform by distillation from the chloroform layer, freeze and crystallize for 2 hours, and filter to obtain 17.1 g (0.059 mol) of atropine crude product ).
[0028] Put 17.1 g (0.059 mol) of the crude product of atropine, 30 ml ethanol, and 150 ml acetone into the reaction bottle, stir, add sulfuric acid dropwise in an ice-water bath at 0-10°C to adjust the pH of the solution to 5-6, freeze overnight to crystallize, filter, 16.8 g (0.051 mol) of atropine sulfate was obtained.
Embodiment 3
[0030] Add 50 ml of ethanol, 200 ml of chloroform, and 20 g (0.06 mol) of α-formylphenylacetic acid tropinate into the reaction flask and stir evenly. Add potassium borohydride 5 (0.09 mol) three times in an ice-water bath at 0-10°C. ) g, stirred and hydrolyzed for 4 hours. Add 200 ml of water to the above mixture, separate the chloroform organic layer, extract the water layer once with chloroform and merge into the original chloroform layer, after the chloroform layer is distilled and recovered chloroform, freeze and crystallize for 2 hours, filter to obtain 16.8 g (0.058 mol) of atropine crude product ).
[0031] Put 16.8 g (0.058 mol) of the crude product of atropine, 30 ml diethyl ether, and 100 ml acetone into the reaction bottle, stir, add sulfuric acid dropwise in an ice-water bath at 0-10°C to adjust the pH of the solution to 5-6, refrigerate overnight for crystallization, and filter. 16.5 g (0.050 mol) of atropine sulfate was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com