Method for preparing silver nanowires
A technology of silver nanowires and silver salts, which is applied in the field of nanomaterials to achieve the effects of high reproducibility, strong controllability, and uniform size and shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 取油酰氯4.4mL溶于20mL甲苯中,2g超支化聚缩水甘油醚溶于20mL吡啶中,所得油酰氯甲苯溶液逐滴滴至超支化聚缩水甘油醚的吡啶溶液中,冰浴搅拌48h后,80℃条件下旋转蒸发除去多余溶剂,经氯仿洗涤去除多余的油酰氯,得到两亲性超支化聚缩水甘油醚。
[0027] 取21mg两亲性超支化聚缩水甘油醚溶于50ml乙二醇中,得混合溶液A。1.6mg 无水氯化铁和0.212g硝酸银依次加入混合溶液A中,搅拌至金属盐完全溶解,得混合溶液C。将混合溶液C转移至水热反应釜中,180℃反应90min,自然冷却后取出粗产物,经丙酮和无水乙醇反复洗涤,6000r / min离心分离产物,得到直径约为120nm的银纳米线。
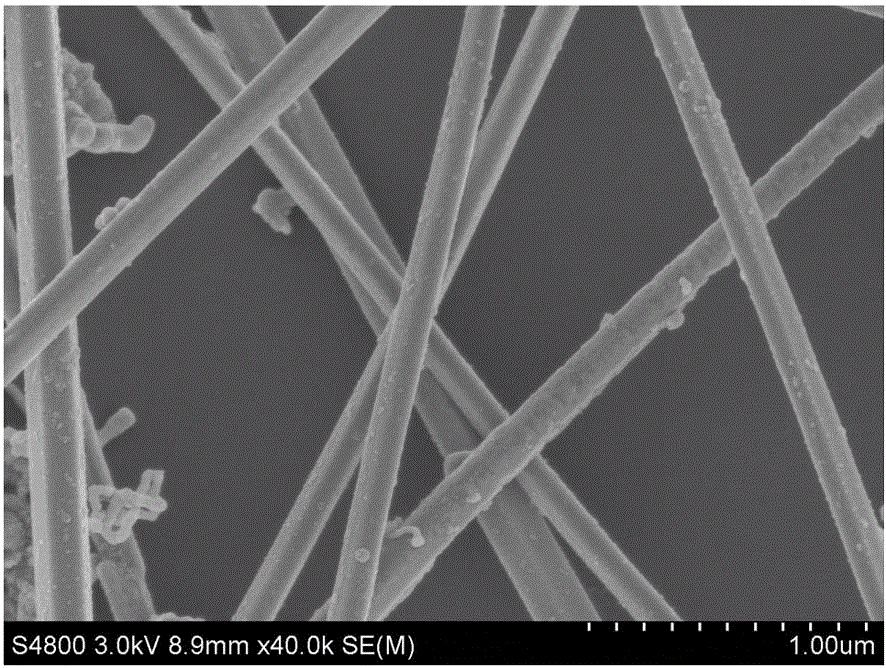
[0028] See attached figure 1 ,是本实施例提供的银纳米线的扫描电镜(SEM)图(放大40K倍)。由图可见,银纳米线的直径在120nm左右,且非常均匀。
[0029] See attached figure 2 ,是本实施例提供的银纳米线的透射电镜(TEM)图。图中银纳米线的直径也在120nm左右,分布均匀,与 figure 1 中SEM显示结果基本一致。
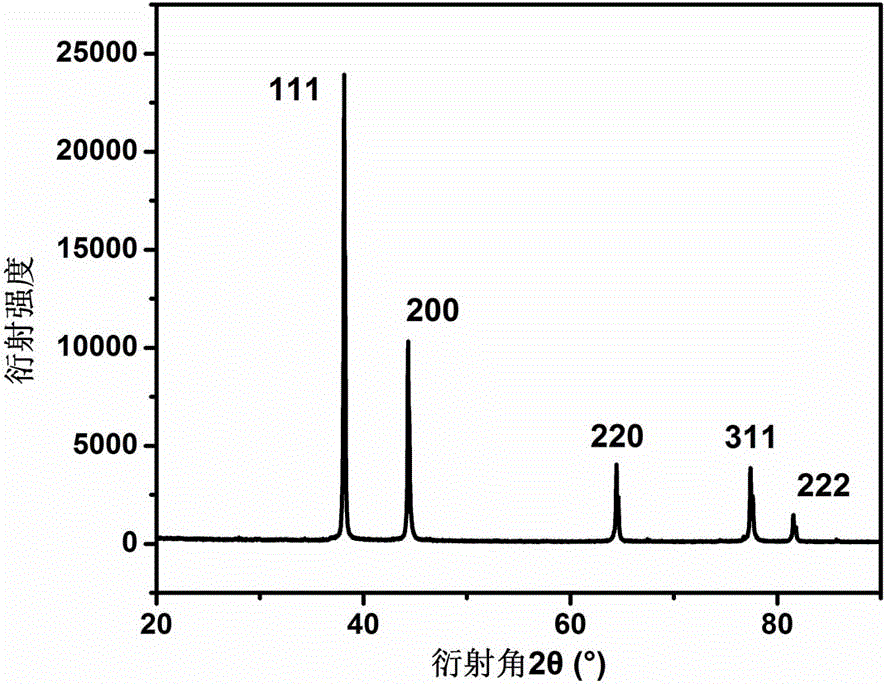
[0030] See attached image 3 ,是本实施例获得的纳米银线的XRD谱图。其 XRD衍射峰与纳米银XRD的标准谱图(JCPDS No.4-0781)完全对应,5个衍射峰分别对应为纳米银的(111), (200)、(220)、(311) 和(222)晶面衍射峰,且银纳米线的 (111) 晶面与 (200)晶面的强度比较高,这意味着银纳米线是沿着 (111) 晶面生长的,表明本方法制备得到的产品确为银纳米线。
Embodiment 2
[0032] 取油酰氯1.0mL溶于60mL甲苯中,1g端氨基超支化聚合物溶于80mL吡啶中,所得油酰氯甲苯溶液逐滴滴至端氨基超支化聚合物的吡啶溶液中,25℃搅拌40h后,80℃条件下旋转蒸发除去多余溶剂,经丙酮洗涤去除多余的油酰氯,得到两亲性端氨基超支化聚合物。
[0033] 取84mg两亲性端氨基超支化聚合物溶于50ml乙二醇中,得混合溶液A。0.8mg 无水氯化铁和0.212g硝酸银依次加入混合溶液A中,搅拌至金属盐完全溶解,得混合溶液C。将混合溶液C转移至水热反应釜中,200℃反应80min,自然冷却后取出粗产物,经氯仿和无水乙醇反复洗涤,6000r / min离心分离产物,得到直径约为200nm的银纳米线。
Embodiment 3
[0035]Dissolve 6.0 mL of oleoyl chloride in 40 mL of ether, 2 g of hyperbranched polyglycidyl ether in 40 mL of methanol, and drop the obtained oleoyl chloride ether solution dropwise into the methanol solution of hyperbranched polyglycidyl ether, and stir for 56 hours under ice bath , rotary evaporation at 50°C to remove excess solvent, and wash with ethyl acetate to remove excess oleoyl chloride to obtain amphiphilic hyperbranched polyglycidyl ether.
[0036] Take 108 mg of amphiphilic hyperbranched polyglycidyl ether and dissolve it in 50 ml of ethylene glycol to obtain mixed solution A. Add 5.6mg of anhydrous ferric chloride and 0.45g of silver nitrate to the mixed solution A in sequence, and stir until the metal salt is completely dissolved to obtain the mixed solution C. The mixed solution C was transferred to a hydrothermal reaction kettle, and reacted at 170°C for 100 min. After natural cooling, the crude product was taken out, washed repeatedly with chloroform and abs...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com