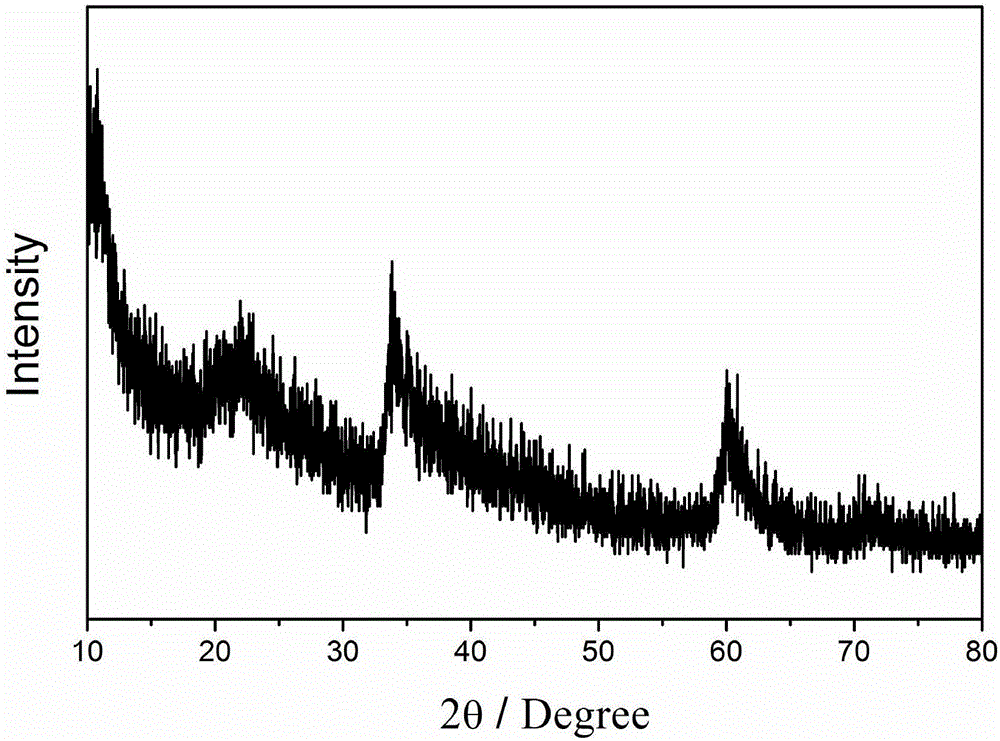
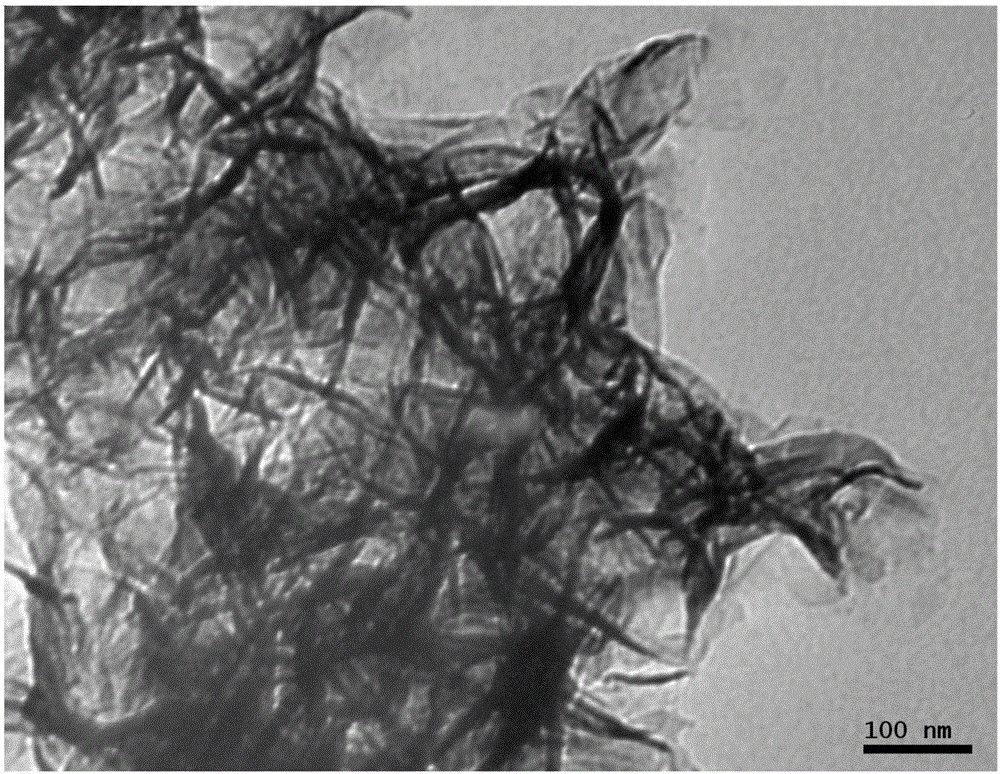
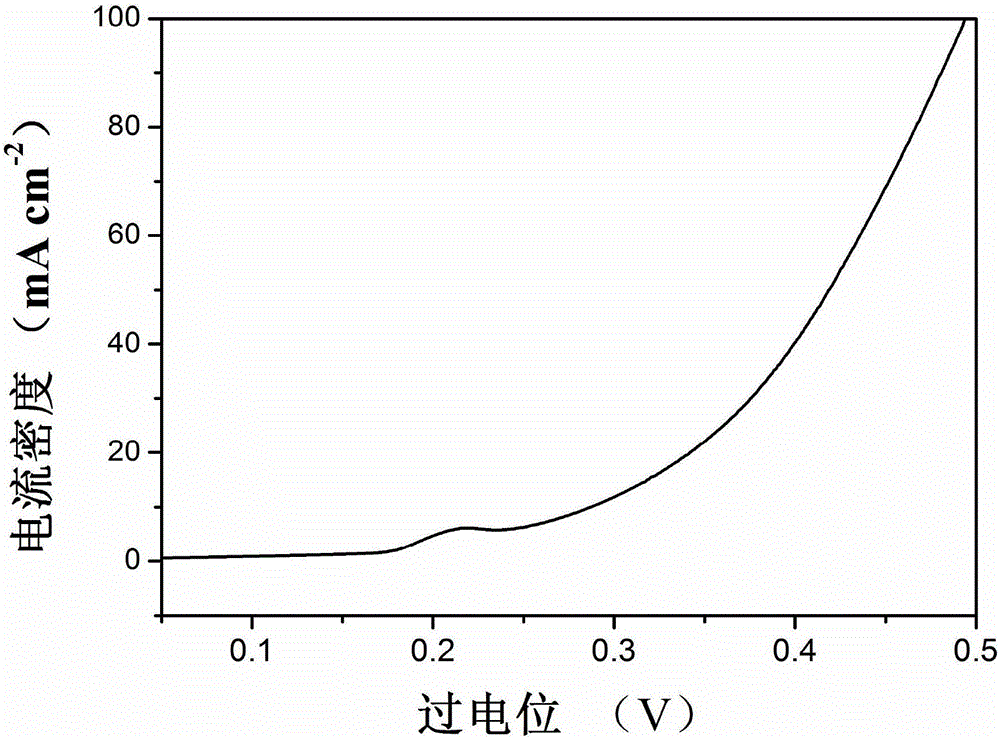
Preparation method of iron-nickel hydrotalcite structure nanosheet for oxygen evolution reaction
An iron-nickel hydrotalcite and oxygen evolution reaction technology is applied in the preparation of graphene-like two-dimensional nanomaterials, and the field of graphene-like two-dimensional materials can solve the problems of poor stability, high overpotential, low current density, etc. Mild conditions, simple operation, good dispersion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Dissolve 1.6 g of nickel chloride and 0.2 g of ferrous oxalate in 80 mL of deionized water, add 0.3 g of sodium tartrate and 3.2 g of potassium hydroxide and continue stirring for 15 to 20 minutes to form a clear solution.
[0024] (2) The solution obtained in step (1) was transferred to a glass vessel, heated and reacted for 30 minutes under the condition of microwave power of 500W, condensed and refluxed, and naturally cooled to room temperature after the reaction was completed.
[0025] (3) Wash the material obtained in the previous step with deionized water and absolute ethanol successively, and then put it into a vacuum drying oven to dry to obtain iron-nickel hydrotalcite structure nanosheets.
[0026] (4) The material synthesized in the previous step is used as a catalytic material for electrolysis of water (oxygen evolution reaction), and the material is dispersed in a mixed solution and nafion, and the ratio of the mixed solution to nafion in the mixed solut...
Embodiment 2
[0029] (1) Dissolve 2.1g of nickel nitrate and 0.3g of ferrous sulfate in a mixed solvent of 60mL of water and 40mL of ethylene glycol, add 1.1g of sodium dodecylbenzenesulfonate and 3.2g of sodium hydroxide to continue Stir for 15-20 minutes to form a clear solution.
[0030] (2) The solution obtained in step (1) was transferred to a glass vessel, heated and reacted at a microwave power of 700W for 15 minutes, condensed and refluxed, and naturally cooled to room temperature after the reaction was completed.
[0031] (3) Wash the material obtained in the previous step with deionized water and absolute ethanol successively, and then put it into a vacuum drying oven to dry to obtain iron-nickel hydrotalcite structure nanosheets.
[0032] (4) The material synthesized in the previous step is used as a catalytic material for electrolysis of water (oxygen evolution reaction), and the material is dispersed in a mixed solution and nafion, and the ratio of the mixed solution and nafion...
Embodiment 3
[0034] (1) Dissolve 2.8g of nickel sulfate and 0.6g of ferrous chloride in a mixed solvent of 30mL of water and 75mL of ethanol, add 0.8g of trisodium citrate and 3.2g of urea and continue stirring for 15 to 20 minutes to form a clear solution .
[0035] (2) The solution obtained in step (1) was transferred to a glass vessel, heated and reacted for 120 minutes at a microwave power of 600W, condensed and refluxed, and naturally cooled to room temperature after the reaction was completed.
[0036] (3) Wash the material obtained in the previous step with deionized water and absolute ethanol successively, and then put it into a vacuum drying oven to dry to obtain iron-nickel hydrotalcite structure nanosheets.
[0037] (4) The material synthesized in the previous step was used as a catalytic material for electrolysis of water (oxygen evolution reaction), and the material was dispersed in a mixed solution and nafion, and the ratio of the mixed solution to nafion in the mixed solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com