Comprehensive utilization method for high-temperature energy
A high temperature and energy technology, applied in the field of comprehensive utilization of high temperature energy, to achieve the effect of improving cleanness or "greenness", improving energy efficiency and reducing pollutant emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
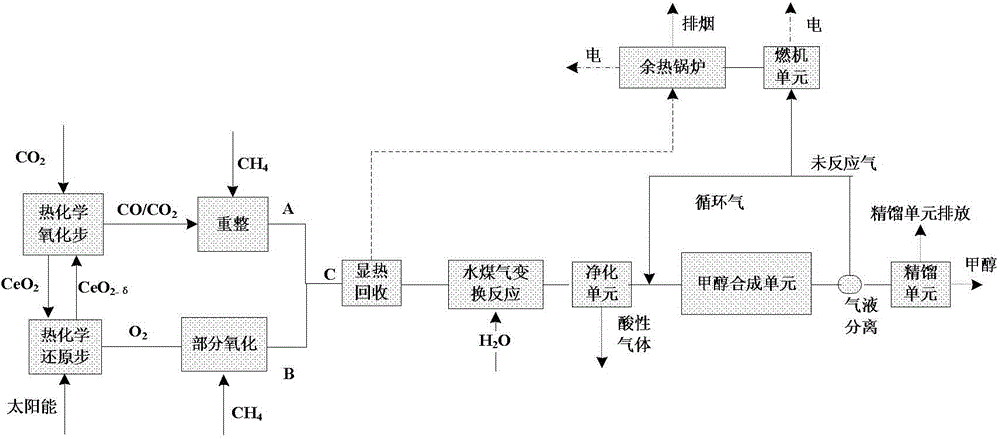
[0069] Such as figure 1 As shown, the two-step decomposition of CO 2 Generate CO / CO 2 The mixed gas, through CH 4 After reforming, the conversion reaction is carried out to achieve a suitable carbon-hydrogen ratio, and methanol is synthesized, while the unreacted gas and waste gas enter the gas-steam combined cycle for power generation.
[0070] The CO added during the two-step thermochemical cycle in this scheme 2 The total amount is 1000kmol / h, and CH is added after the reduction reaction 4 The amount of partial oxidation is 42kmol / h, the H produced at this time 2 The ratio of mole fraction to CO is exactly 2:1, figure 1 Shown at point B in the middle; CO / CO at the outlet of the two-step oxidation step 2 The mixed gas is added to CH 4 carry out the reformation reaction. The total methane input flow includes reformed methane flow and partial oxidation methane flow. The key point parameters in the scheme are shown in Table 2.
[0071] Table 2 Main parameter settings ...
Embodiment 2
[0083] Figure 4 is a two-step decomposition of H 2 O methanol power polygeneration system diagram, two-step decomposition of water vapor, the product is H 2 and H 2 O mixture. If in 1000kmol of H 2 with H 2 180kmol / h of CH was passed into O mixed gas 4 , and the O generated in the two-step reduction step 2 with CH 4 undergo partial oxidation. The reacted gas is dehydrated and then pressurized to synthesize methanol, and the unreacted gas is passed into the gas steam cycle to perform work. At this time, the solar heat consumption of methanol is 120.54GJ / t, and the conversion efficiency of solar energy to methanol energy is 19.48%. Compared with Kim et al.'s 2012 high-temperature solar thermochemical cycle dual-temperature water splitting efficiency of 9.3%, the solar methanol conversion efficiency has doubled.
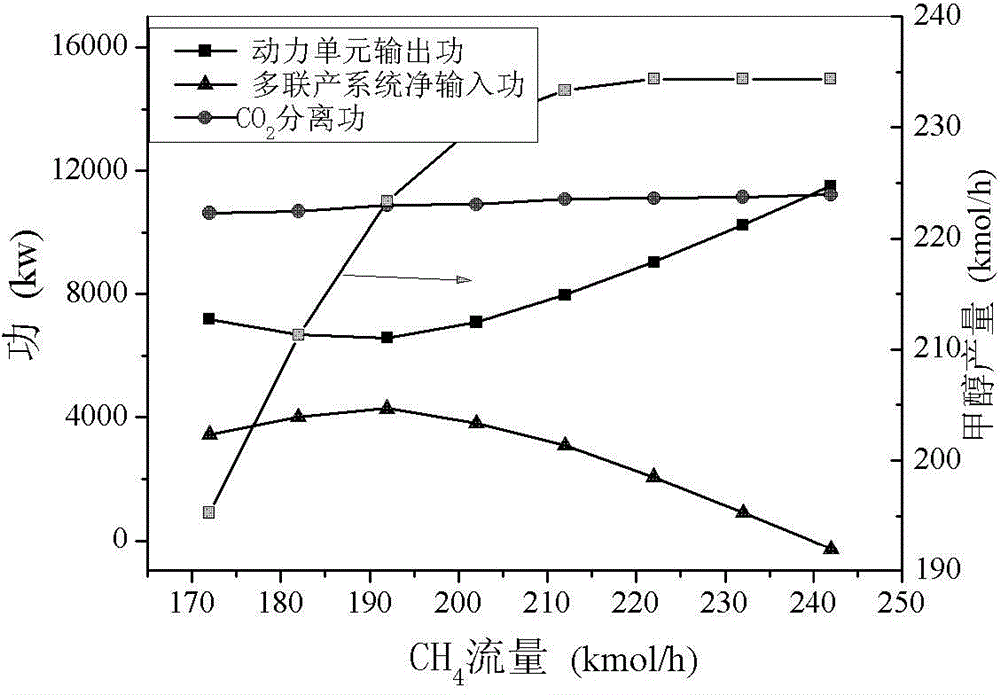
[0084] By changing the access to the CH 4 Total amount, from 142-192kmol / h, it can be found that the energy consumption varies around 130GJ / t, while the con...
Embodiment 3
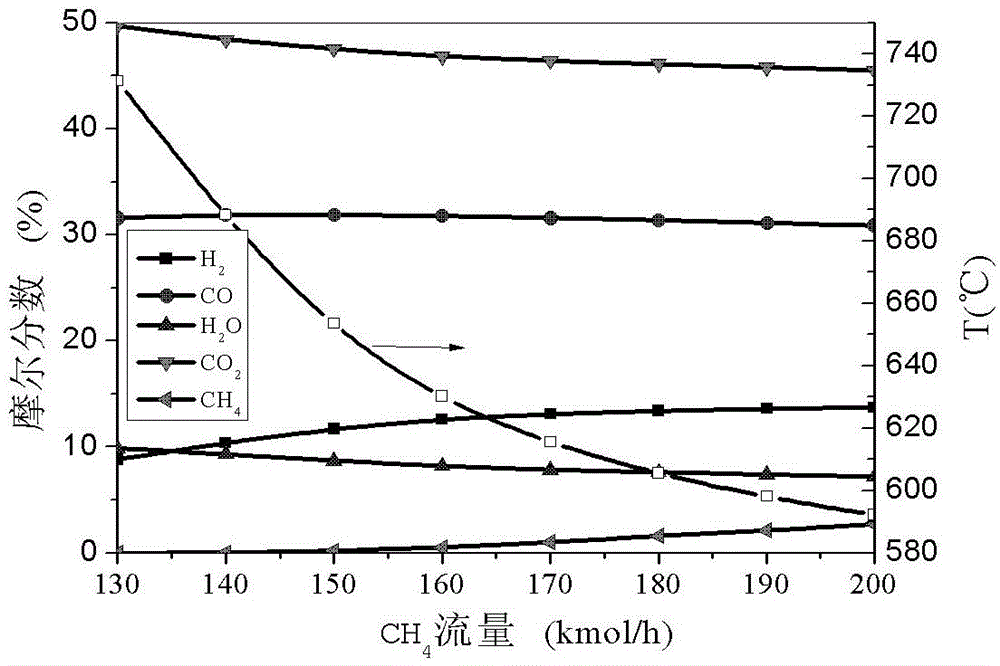
[0087] By analyzing Example 1 and Example 2, it can be found that the two-step method decomposes H 2 After O is added to complement methane, H 2 The mole fraction of CO is much higher than that of CO; while the two-step method decomposes CO 2 After adding methane for complementation, the proper carbon-to-hydrogen ratio is still not achieved for methanol synthesis, and a water-gas shift reaction is required. Therefore we consider combining Example 1 and Example 2 to get Figure 5 Example 3 shown. via the entrance to the CO 2 and H 2 The amount of O and methane is adjusted so that Figure 5 The ratio of carbon to hydrogen in the mixed gas at point D is 1:2. Here, we will CO 2 The flow rate is taken as 1000kmol / h, H 2 The flow rate of O is taken as 1400kmol / h, and can be obtained by adjusting the input flow rate of methane Figure 5 Component mole fraction of point D in middle and the change curve of mixing temperature ( Figure 6 (a)). CO 2The mole fraction of is abo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com