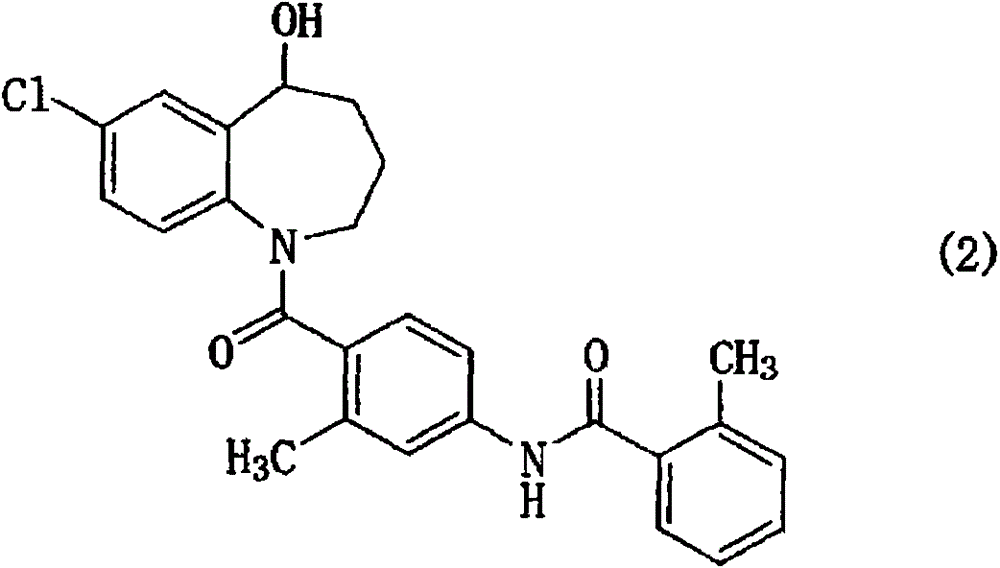
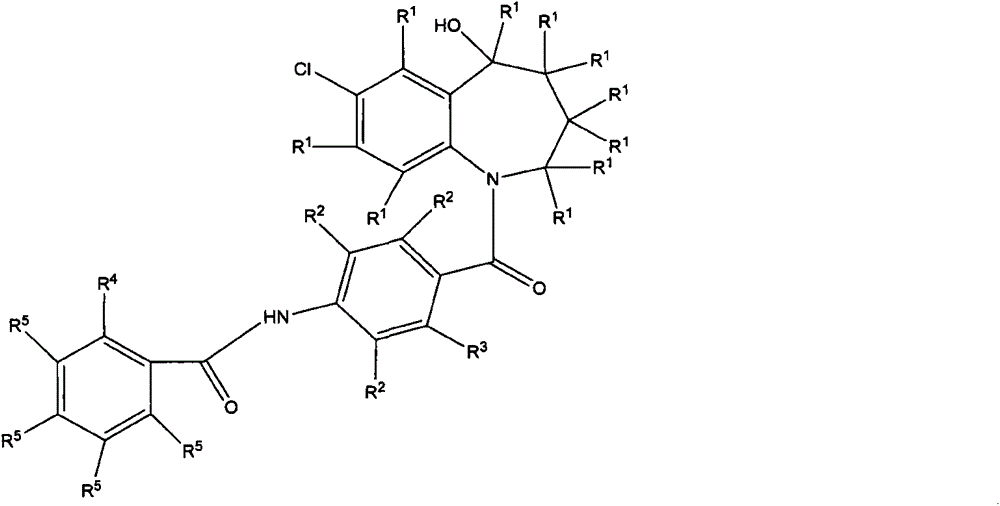
Benzazepine compound
A kind of technology of hetero compound and benzazepine, applied in the field of benzazepine hetero compound, can solve the problems such as undisclosed deuterium compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0062] N-(4-(7-chloro-5-oxyl-2,3,4-trihydro-1H-benzo[b]azepine -1-carbonyl)-3-methylphenyl)-2-methylbenzamide preparation
[0063] To N-(4-(7-chloro-5-hydroxyl-2,3,4,5-tetrahydro-1H-benzo[b]azepine - Manganese dioxide (2 g) was added to a suspension of 1-carbonyl)-3-methylphenyl)-2-methylbenzamide (2 g) in dichloromethane (40 mL), and refluxed for 7 hours. After cooling the reaction mixture, it was filtered through celite, washed with dichloromethane, and purified by silica gel column chromatography (n-hexane:ethyl acetate=10:1→3:1) to obtain 0.94 g of the title compound. Properties: colorless amorphous powder
[0064] 1 H-NMR (CDCl 3 )δppm
[0065] 1.91-1.31(2H, m), 2.43(3H, s), 2.49(3H, s), 2.89(2H, t, J=6.3Hz), 3.30-4.60(2H, m), 6.48-7.00(2H, m), 7.01-7.70 (8H, m), 7.78 (1H, s).
Embodiment 1
[0067] N-(4-(7-chloro-5-hydroxy-2,3,4-trihydro-5-deutero-1H-benzo[b]azepine -1-carbonyl)-3-methylphenyl)-2-methylbenzamide preparation
[0068] At 0°C, N-(4-(7-chloro-5-oxyl-2,3,4-trihydro-1H-benzo[b]azepine -1-carbonyl)-3-methylphenyl)-2-methylbenzamide (0.4g) in deuterated methanol (10mL) solution was added sodium borodeuteride (Sodium borodeuteride) (0.045g), in the same Stir at temperature for 2 hours. Heavy water (2 mL) was added to the resulting reaction mixture, and after stirring for 10 minutes, water was added and extracted with ethyl acetate. The resulting aqueous layer was extracted again with ethyl acetate. The resulting ethyl acetate layers were combined, dried over anhydrous magnesium sulfate, and the solvent was distilled off, and the residue was recrystallized from acetone-diethyl ether to obtain 0.35 g of the title compound.
[0069] Yield: 87%
[0070] Appearance: white powder
[0071] 1 H-NMR (DMSO-d6, 80°C) δppm
[0072] 1.40-2.19(4H, m), 2.36(3H,...
Embodiment 2
[0076]
[0077] N-(4-(7-chloro-2,3-dihydro-5-hydroxy-4,4,5-trideutero-1H-benzo[b]azepine -1-carbonyl)-3-methylphenyl)-2-methylbenzamide preparation
[0078] To N-(4-(7-chloro-5-oxyl-2,3,4,5-tetrahydro-1H-benzo[b]azepine -1-carbonyl)-3-methylphenyl)-2-methylbenzamide (300mg) in deuterated methanol (10mL) solution was added 0.05M sodium hydroxide deuterated methanol solution (13μl), under argon atmosphere Stir at room temperature. After stirring for 16 hours, use 1 H-NMR confirmed that the 4-position proton disappeared, and sodium borodeuteride (0.037 g) was added to the reaction liquid at 0°C, and stirred at the same temperature for 2 hours. Heavy water (2 mL) was added to the resulting reaction mixture, and after stirring for 10 minutes, water was added and extracted with ethyl acetate. The resulting aqueous layer was extracted again with ethyl acetate. The resulting ethyl acetate layers were combined, dried over anhydrous magnesium sulfate, and the solvent was distill...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com