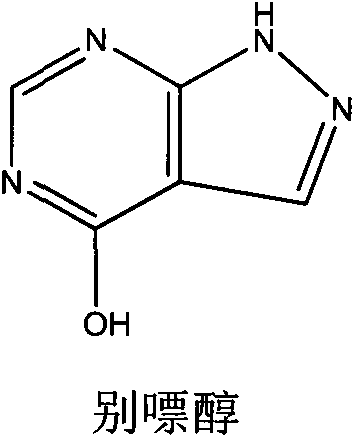
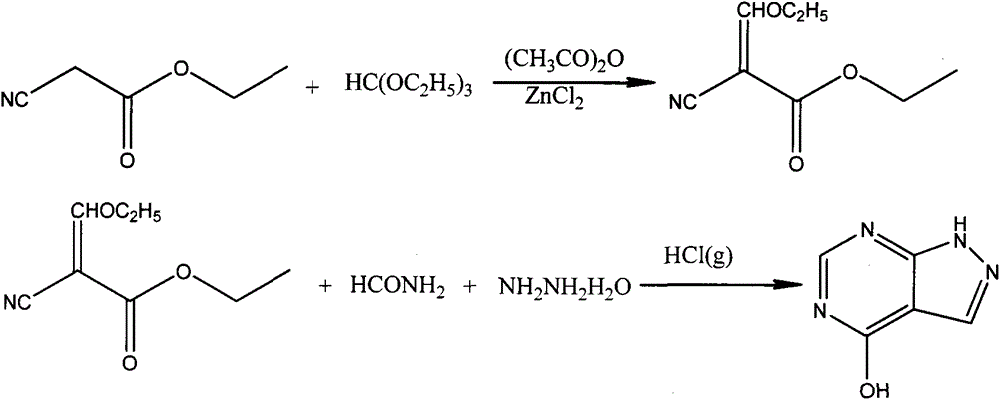
Method for carrying out solvent-out crystallization on allopurinol
A technology of dissolution crystallization and allopurinol, which is applied in the fields of medicine and chemical industry, can solve the problems that the product department is easy to meet the requirements of medicine, low equipment production efficiency, and high organic residue, so as to achieve less solvent residue, good product quality, and increase production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Add 100 g of crude allopurinol to 1000 g of sulfolane, heat up to 98° C. under stirring to dissolve the material, and obtain a solution. 200 g of purified water was added to the solution for crystallization, and the filter cake was washed twice with 30 g of purified water, sucked dry, and dried at 80°C to obtain 89.2 g of a white crystalline product.
[0036] Experimental results: The HPLC normalization method purity of the product is 99.92%, the yield is 89.2%; the maximum simple impurity content is 0.04%, and other quality indicators meet the requirements of the 2010 edition of "Chinese Pharmacopoeia".
Embodiment 2
[0038] Add 100 g of crude allopurinol to 650 g of sulfolane, heat up to 148° C. under stirring to dissolve the material, and obtain a solution. 200 g of purified water was added to the solution for crystallization, and the filter cake was washed twice with 30 g of purified water, sucked dry, and dried at 80°C to obtain 89.6 g of a white crystalline product.
[0039] Experimental results: The purity of the product by HPLC normalization method is 99.90%, the yield is 89.6%; the maximum simple impurity content is 0.05%, and other quality indicators meet the requirements of the 2010 edition of "Chinese Pharmacopoeia".
Embodiment 3~6
[0041] Based on Example 2, by only changing the consumption of purified water, other conditions are constant (the following table uses "—" to indicate that this condition is the same as Example 2), the results obtained are as follows:
[0042] sequence
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com