Fused antibacterial peptide and preparation method thereof
An antibacterial peptide, pet-22b-yselp technology, which is applied in the field of fusion antibacterial peptides and their preparation, can solve the problems such as the lack of lethality of host bacteria, and achieve the effect of solving large-scale application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the preparation of fusion antimicrobial peptide
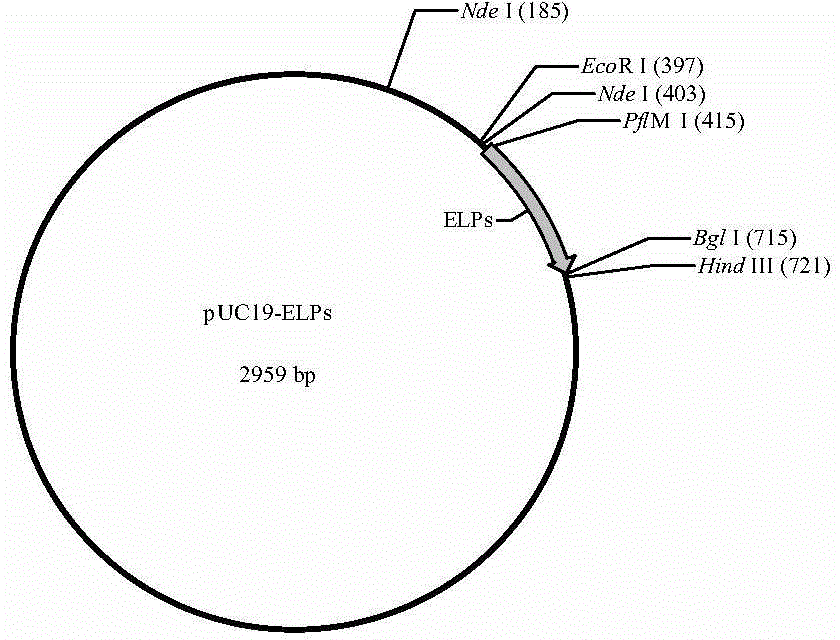
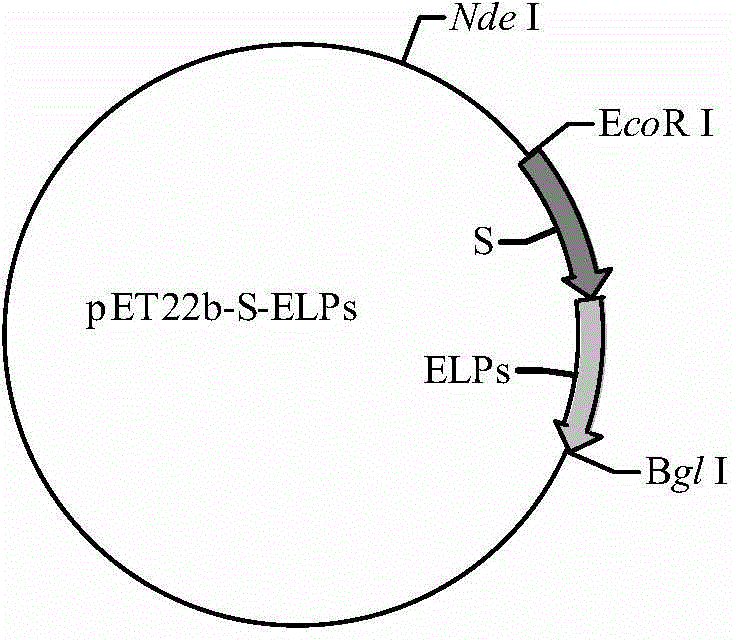
[0045] 1) Construction of pET-22b-YSELP expression vector: According to the amino acid sequence of the antibacterial peptide YFGAP (as shown in SEQ ID NO.4), the gene sequence of the antibacterial peptide YFGAP was designed with E.coli standard codon optimization, and the gene sequence The 5' end introduces the Nde I restriction site, and the 3' end introduces the EcoR I restriction site, and chemically synthesizes the gene sequence shown in SEQ ID NO.5; the synthesized gene is double-digested with Nde I and EcoR I respectively The sequence was connected to the pUC19 plasmid that had been digested with the same double restriction enzymes by T4 DNA ligase and transformed into E.coli DH5α competent cells (refer to the "Molecular Cloning Experiment Guide" for specific transformation conditions), and the screened positive clones were provided by Sangon Bioengineering (Shanghai) Co., Ltd. sequenced, and the corr...
Embodiment 2
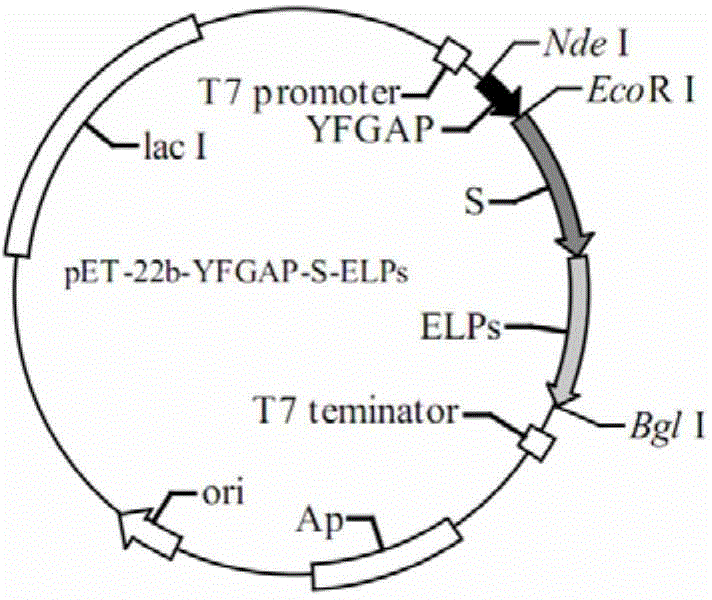
[0054] Embodiment 2: Identification of expression vector pET-22b-YSELP
[0055] The operation is the same as step 1) of Example 1, transform pET-22b-YSELP into E.coli BL21(DE3) competent cells, apply LB plates containing 50 μg / mL ampicillin (Amp) to screen positive clones, and pick Insert a single colony into LB liquid medium, culture at 37°C, 200r / min for 12 hours, extract plasmid DNA (i.e. expression vector pET-22b-YSELP), and verify by Nde I and Bgl I double enzyme digestion, the results are as follows Image 6 As shown, the results of agarose gel electrophoresis show that the molecular weight of the enzyme-cut bands is consistent with the size of the carrier gene, and the three bands are 2583, 2050, and 1502bp respectively; the sequencing results of Sangon Bioengineering (Shanghai) Co., Ltd. also show that the gene sequence is correct , the recombinant expression vector was constructed successfully.
Embodiment 3
[0056] Example 3: Effect of YSELP expression on the growth of host bacteria E.coli BL21(DE3)
[0057] E.coli BL21(DE3) / pET-22b-YSELP was inoculated into LB medium containing 100μg / mL ampicillin at a ratio of 1:100, cultured overnight at 37°C, 200r / min, and inoculated into TB medium, 37°C, 200r / min Cultivate for 4 hours, add IPTG to induce, take the bacterial solution every 1 hour (every 2 hours after 12 hours) to measure the OD value at 600nm; use uninduced E.coli BL21(DE3) / pET-22b-YSELP as the control.
[0058] The result is as Figure 7 As shown, the growth of the host strain E.coli BL21(DE3) was inhibited after adding IPTG to induce expression. After 8 hours of culture, the turbidity of the bacterial solution began to decrease. After 12 hours, the turbidity of the bacterial solution gradually increased, and the concentration of the bacteria increased. E.coli BL21(DE3) grew rapidly again, indicating that the expression of YSELP protein did not completely kill the host itsel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com