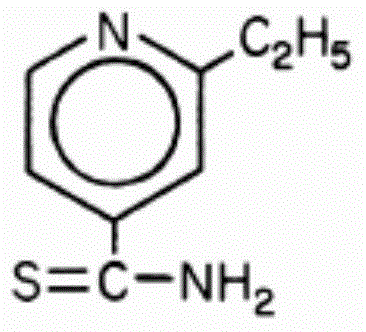
Preparation method of ethionamide tablet
A technology of ethionamide tablets and ethionamide, which is applied in the directions of pharmaceutical formulations, antibacterial drugs, active ingredients of heterocyclic compounds, etc. and other problems, to achieve the effect of quick onset, beneficial to the body's absorption and bioavailability, and avoiding cumbersome processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] For every 1,000 ethionamide tablets, weigh the materials according to the following formula:
[0049] Tablet core prescription:
[0050]
[0051] Coating liquid prescription:
[0052] Opadry 8.0g
[0053] 95% ethanol 100mL
[0054] Make about 100mL and add water to dilute to 8% solution
[0055] Preparation Process:
[0056] 1. After pulverizing the ethionicotinamide solid dispersion, pass through a 60-mesh sieve, and pass the microcrystalline cellulose, povidone, and croscarmellose sodium through an 80-mesh sieve for use;
[0057] 2. Weigh the prescription amount of ethionicotinamide solid dispersion and croscarmellose sodium, add them to the V-type mixer and mix for 5 minutes, then add the prescription amount of microcrystalline cellulose and povidone and mix for 5 minutes;
[0058] 3. Add the mixture of step 2 to the dry granulator, adjust the pressure of the hydraulic wheel to 1.8MPa and the speed of 10r / min to press it into a thin strip of 3mm thick, which is crushed into small ...
Embodiment 2
[0064] For every 1000 ethionamide tablets, weigh the materials according to the following formula:
[0065] Tablet core prescription:
[0066]
[0067] Coating liquid prescription:
[0068] Opadry 8.0g
[0069] 95% ethanol 100mL
[0070] Make about 100mL and add water to dilute to 8% solution
[0071] Preparation Process:
[0072] 1. After crushing the ethionicotinamide solid dispersion, pass it through a 60-mesh sieve, and pass the microcrystalline cellulose, Ludipress LCE, and cross-linked polyvinylpyrrolidone through an 80-mesh sieve, respectively, for use;
[0073] 2. Weigh the prescription amount of ethionicotinamide solid dispersion and micronized silica gel, add them to the V-type mixer and mix for 5 minutes, then add the prescription amount of microcrystalline cellulose, Ludipress LCE, and cross-linked polyvinylpyrrolidone and mix for 5 minutes;
[0074] 3. Add the mixture of step 2 to the dry granulator, adjust the pressure of the hydraulic wheel to 1.5MPa and the speed of 12r / min ...
Embodiment 3
[0080] For every 1000 ethionamide tablets, weigh the materials according to the following formula:
[0081] Tablet core prescription:
[0082]
[0083]
[0084] Coating liquid prescription:
[0085] Opadry 8.0g
[0086] 95% ethanol 100mL
[0087] Make about 100mL and add water to dilute to 8% solution
[0088] Preparation Process:
[0089] 1. After crushing the ethionicotinamide solid dispersion, pass it through a 40-mesh sieve, and pass the microcrystalline cellulose, povidone and sodium carboxymethyl starch through a 60-mesh sieve for use;
[0090] 2. Weigh the prescription amount of ethionicotinamide solid dispersion and sodium carboxymethyl starch, add to the V-type mixer and mix for 5 minutes, then add the prescription amount of microcrystalline cellulose and povidone, and mix for 5 minutes; add 1 / 2 The prescription amount of magnesium stearate is mixed for 3 minutes;
[0091] 3. Add the mixture of step 3 to the dry granulator, adjust the pressure of the hydraulic wheel to 1.7MPa and...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap